Dairy & Veterinary Sciences - Juniper Publishers
Abstract
Bovine viral diarrhea virus is a pest virus that can cause severe clinical signs; Major economic losses continue to result from reproductive losses and exacerbation of concurrent bacterial or viral infections. Of less clinical importance is severe clinical disease due to BVDV alone, either as result of acute infection by highly virulent isolate or by development of mucosal disease in persistently infected calves. Disease due to BVDV can arise at any stage of life and is often dependent on the virulence of infecting isolate. Survival of BVDV virus in population depended up on the characteristics of persistently infecting its host. Persistent infection arises by the unique ability of the BVDV to survive by immune tolerance in bovine fetus through evasion of both innate and acquired immunity in utero. Adaptive immunity is avoided by immune tolerance through infection of fetus prior to development and maturation of adaptive immunity. The pathogenesis of BVDV infection is complex, with infection pre- and post-gestation leading to different outcomes. Infection of the dam during gestation results in fetal infection, which may lead to embryonic death, teratogenic effects, or the birth of persistently infected, (PI) calves. PI animals shed BVDV in their excretions and secretions throughout life and are the primary route of transmission of the virus. These animals can usually be readily detected by virus or viral antigen detection assays (RT-PCR, ELISA), except in the immediate post-natal period where colostral antibodies may mask virus presence. Acute infection with BVDV results in transient viraemia prior to seroconversion and can lead to reproductive dysfunction and immunosuppression leading to an increased incidence of secondary disease. Antibody assays readily detect virus exposure at the individual level and can also be used in pooled samples (serum and milk) to determine herd exposure or immunity. Diagnostic tests can be used to diagnose clinical cases, establish disease prevalence in groups and detect apparently normal but persistently infected animals.
Keywords:Bovine viral diarrhea; Mucosal disease; Persistent infection; Virus
Abbreviations:BVD: Bovine Viral Diarrheal; BVDV1: Bovine Viral Diarrhea Virus type1; BVDV2: Bovine Viral Diarrhea Virus type2; BVDV: Bovine Viral Diarrheal Virus; Cp: Cytopathogenic; CSFV: Classical Swine Fever Virus; ELISA: Enzyme Linked Immune Sorbent Assay; FCS: Fetal Calf Serum; FCS-SM: Fetal Calf Serum Supplemented Media; IHC: Immune Histocompatibility; MD: Mucosal Diseases ; MLV: Modified Live Vaccine NCP: Non cytopathogenic; OIE: Office of International Epizootics; PI: Persistently Infected; RNA: Ribonucleic Acid; RT-PCR: Reverse Transcriptase Polymerase Chain Reaction; VI: Virus Isolation; VN: Virus Neutralizations
Introduction
Bovine viral diarrhea virus (BVDV) is a Pest virus of the family Flaviviridae capable of causing serious clinical diseases in cattle [1]. The virus is divided into two genomes (BVDV-1 and BVDV-2) because of antigenic and genetic differences [2]. And Infection with BVDV is also known to have significant financial impact stemming primarily from the reproductive and immunosuppressive effects of acute infection [3]. As such, control and eradication programs are becoming increasingly common across much of the cattle-producing world including legislated, regional schemes and voluntary, herd-based schemes [4]. While healthy, immune-competent cattle (or late-term, immune-competent fetuses) may suffer from acute BVDV infection resulting in sero-conversion; the disease is primarily spread and maintained in cattle populations by persistently infected (PI) individuals that Persistency of infection arises from fetal infection in early gestation Following acute infection in the dam [5]. Most control programs aim to eliminate PI cattle and hence the source of continuing infection [4].
This requires the efficient and effective use of accurate diagnostic tests, and a wide range is readily available for the detection of BVDV virus, antigen (Ag) or specific antibodies (Ab) that the tests will return varying results depending on the current or historical BVDV infection status and Animals that have never been exposed to the virus will test negative for Ab, Ag, and virus with the broad nature of disease, transmittance, and lack of treatment made BVD global pandemic and one of the most significant cattle diseases in world Coupled with vast clinical symptom and associated death loss, gravity of BVD makes it a disease no longer overlooked [6]. The overall effects of BVD are difficult to examine, due to the breadth of the disease. Research and data highlighting effects of the disease is very limited, simply since too many variables are present for accurate measurement. Furthermore, if considered the worldwide prevalence of BVD, it is easily understood why BVD is an important cattle disease from an economic standpoint [7].
The areas affected by BVD, specifically reproduction, production and immunosuppression, are what make it such an expensive disease; Reproductive disorders & syndromes associated with BVDV include: abortion, early embryonic loss, fetal defects, conception failure, normal to prolonged returns to estrous, increase in return to service numbers, spread out calving pattern, slow to grow and weak calves, birth defects such as blind calves or calves with neurological problems, poor weaning rates due to ill thrift and death and A good pregnancy rate does not indicate a BVDV free herd as reproductive losses is associated with the timing of infection [4].
Production effects span those regarding decline in feed to gain efficiency, lower carcass quality, and decreased milk production and because of the vague and diverse clinical signs observed with the disease, it is very difficult to clinically diagnose BVD infected cattle. The difficulty of clinically diagnosing BVD positive cattle impedes the studying of these cattle in a clinical setting. Regardless, understanding the nature of viral infections in animals it should be noted that the side effects are not usually contained to one specific area of the body. A virus such as BVD has been noted to have potential complications with every body system in cattle. Although rare, BVD viruses have manifested in many ways as rare as causing skeletal deformities to severe neurological damage (Houe, 1994). Finally, immunosuppression may also lead to secondary disorders, along with a whole host of other [8].
With above background, the objectives of this paper are:
To provide a brief review on the economic importance of
BVD in production and reproduction.
To know the management systems of BVD infection
during any outbreak time and infectious period.
To understand the nature and characteristics of BVD.
To provide information on the epidemiology of BVD.
To emphasize diagnostic, prevention and control
methods of BVD.
General Overview
Etiology
BVDV is a member of the pest virus genus caused by a virus called Bovine Viral Diarrhea Virus (BVDV) that there are several ways in which BVDV infection can manifest in a herd and cause economic loss of that the most important craffied one’s are the immunosuppressive effects, reproductive losses and losses associated with persistently infected animals (Becher et al., 2011).
Viral taxonomy
There are four recognized species within the pest virus genus. These species are BVDV-1, BVDV-2, border disease virus of sheep and classical swine fever virus, previously known as hog cholera virus [9]. (See box below)
Epidemiology The occurrence of BVD infection
Acute infection is the result of horizontal transmission to a susceptible animal following contact either with an acutely infected animal that is shedding BVDV, or with a PI animal that sheds BVDV continuously that it may also be acquired through several routes of transmission such as inhalation, ingestion and transplacental [10].
The spread of BVD
The greatest period of spread is during yarding, particularly when cattle from different groups or herds are mixed or penned in adjacent pens [11]. And the main source of infection is persistently infected (PI) animals that shed many viral particles in all body fluids and excretions (milk, urine, feces, saliva). Infection in susceptible animals tends to occur from direct animal to animal contact that may occur during yarding, mustering, agricultural shows and sales and the most common ways that BVDV is introduced to a herd through the introduction of replacement breeding animals, new bulls and occasionally over the fence contact with infected animals [3].
Persistence of BVD
Persistently infected (PI) calves are created when a fetus is exposed to BVDV during the first half of gestation that on this time, the fetal immune system is not developed enough to respond to a BVDV infection so the fetus might be aborted, but if the fetus survives it will likely develop into a PI calf. Some PI calves grow poorly while others may look healthy and grow very well, making it impossible to consistently detect PI animals visually. Most PI animals die by 2 years of age, but some will survive for several years and constantly shed BVDV throughout their life (Givens et al, 2010).
PI cattle are much more efficient at transmitting the disease due to the higher amount of systemically circulating virus present within the animal. With higher amounts of circulating virus these animals will secrete higher levels of the virus. Additionally, PI cattle will secrete the virus in various forms from inoculation of the virus until their death. The potency of transmission is of major concern, and it has been shown that horizontal transmission from a PI animal to a seronegative animal can be detected within 20 hours after direct contact [9] (Figure 1).
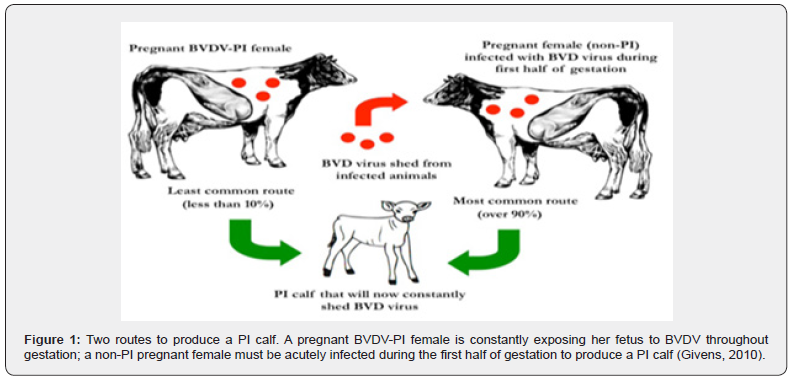
Transmission and source of infections Transmission
Bovine pest virus is spread by close contact (nose-to-nose) between cattle. The greatest period of spread is during yarding, particularly when cattle from different groups or herds are mixed or penned in adjacent pens [11]. BVDV does not usually survive in the environment very long (less than 3 weeks), so direct transmission between animals is the most common route of transmission that Acutely infected animals are a temporary source of BVDV transmission, but PI animals shed millions of viral particles every day; therefore, serve as a constant source of BVDV exposure in a herd because they continuously shed virus in saliva, mucous, tears, milk, feces, urine, and any other bodily secretion and its transmission may occur vertically (i.e., before birth), leading to congenital infection of the fetus, or the transmission may occur horizontally (i.e., after birth) that Congenital infections may cause resorption, abortion, still birth, or live-birth and Congenitally infected fetuses that survive in utero infection (i.e., the live-births) may be born as BVDV-infected calves [12] (Figure 2).
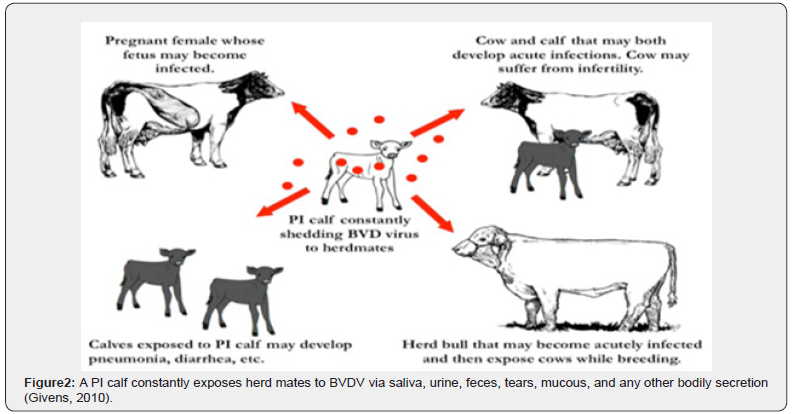
Source of infection
Persistently infected carrier calves play a key role in spreading the virus as they can shed viruses throughout their life, acting as a source of infection for other animals. This most commonly occurs when these cattle are between 6–24 months of age. However, some PI’s have been known to survive and enter the breeding herd. They may die from a range of diseases caused by the immunosuppressive effects of BVDV infection and occasionally they may develop Mucosal Disease [13].
Risk factors and host range Risk factors for BVDV infections.
The risk factors for BVDV infections are often a reflection of the risk of direct or indirect contact with PI cattle. The suspected risk factors include livestock trade, pasturing animals, use of common pasture for infected and susceptible cattle, cattle density, number of infected neighboring herds, broken fences, animal contact between herds (e.g., livestock exhibitions), weak hygiene, and presence of other susceptible ruminants, such as sheep and wildlife [14].
Host range
Other than cattle carrying the BVD virus, domesticated farm animals have been shown to have the potential of becoming infected with BVD as well. So, the virus has been found in sheep, pigs, and wildlife, although the chance of transmission to or from cattle has not been fully established but transmission between sheep and cattle has been experimentally proven [15]. The BVD virus has been found in pigs as classical swine fever virus (CSFV), but once again the transmission to cattle is considered doubtful. Initially this was thought to be a possible mode of transmission to and from cattle, and some even thought that the disease may have originated from wildlife populations such as serological evidence exists that deer can become infected with BVD [16].
Pathogenesis
BVDV infection has a wide range of clinical presentations and unique diagnostic challenges; so, to select an appropriate diagnostic test, it is important first to understand the pathogenesis of the disease. BVDV is divided into noncytopathogenic (ncp), and cytopathogenic (cp) biotypes based on effects on cultured cells rather than in the infected host. Cp biotypes induce apoptosis in cultured cells while ncp biotypes do not [17]. Non-cytopathogenic BVDV, however, appears to be the cause of acute infections and can be transmitted in a wide range of body fluids, including nasal discharge, urine, milk, semen, saliva, tears, and fetal fluids [18]. Also, Cp BVDV has been shown to be capable of inducing acute infection under experimental conditions [19].
Clinical Signs and symptoms Clinical signs
Clinical signs of infected cattle cover a wide spectrum from showing no signs to death. The varying clinical signs depend on what form of the virus the animal is carrying along with the extent of the infection. Cytopathic strains of BVD will result in more severe reproductive effects including embryonic loss, stillbirths, and congenital defects. Additionally, it forms of the virus contracted in older cattle will cause these animals to present themselves critically in a clinical setting causing fever, depression, lack of appetite, labored breathing, oral ulcerations, and diarrhea to be observed also in the most severe cases Bleeding of internal organs and death has been observed. Supportive care such as fluids and anti-inflammatory drugs may be used, but the likelihood of losing the animal is still relatively high. Noncytopathic strains of the virus will cause infected cattle to show mild clinical signs if any at all. So, this type of virus will create PI calves if they are infected in utero and will cause transiently affected animals if contracted by older animals. In general, a noncytopathic form of the virus will have less detrimental effects than a cytopathic strain of the virus [20].
Secondary Diseases
Other than the consequences of reproductive disorders, Bovine Respiratory Disease (BRD) is a major disease that is often coupled with BVD. It is much like BVD, an umbrella term for a wide range of bacterial and viral caused respiratory diseases. So, those that contained high levels of BVD seropositive cattle were at a higher risk of Bovine Respiratory Syncytial Virus (BRSV) [14]. Viruses are the principle pathogenic instigators of BRD, making prevention of BVD key to keeping BRD herd levels at a minimum and should be noted that immunosuppression is not the only explanation for BRD being a secondary disease to BVD (Hansen et al, 1996).
Clinical syndromes associated with BVD.
There is a wide spectrum of outcomes associated with BVDV that its Infections may be subclinical (no outward signs of disease), clinical or chronic. This depends on the immune status of the animal, the stage of gestation of female animals and the amount or strain of virus that the animal is exposed to. Common outcomes of exposure to BVDV in cattle include one or more of the following Reproductive Syndromes such as embryonic loss, stillbirths, and congenital defects the most common once; conception failure, normal to prolonged returns to estrous, increase in return to service numbers, spread out calving pattern, slow to grow and weak calves; birth defects such as blind calves or calves with neurological problems, poor weaning rates due to ill thrift and death [14].
Pathology
Pathologically, PI cattle are viremic, yet clinically they often show little or no signs of being infected. The classical PI animal is the one that is never shows signs clinically and may seem fine but is the silent culprit for regenerating the disease; so, these reasons that PI animals are of greatest concern to producers. The common pathology of the disease is cerebral hypoplasia, ulcerative esopagitis, hemorrhagic necrosis of peyers patchs on ileum, colitis and crypt dilatation unilateral corneal opacity are good examples of BVD [21] (Figure 3(a,b,c)).
Diagnostics
In general, diagnostic assays for detection of BVDV infections can be divided into Serological methods, i.e. methods aimed at detection of virus-specific antibodies as an indication of exposure to the pathogen, and methods aimed at detection of infectious virus or viral components (antigen or nucleic acids), as an indication of current infection [22].
Serological methods
In the context of BVDV control, the main objectives of serological methods are to Differentiate between exposed and non-exposed herds, to monitor progress and Drawbacks within an ongoing control programme, and to investigate immune status in individual animals in infected herds, to identify possible PI animals. A PI animal is, as previously mentioned, generally seronegative, unless it has persisting maternal antibodies, or has been exposed to and infected with a heterologous strain. Serological methods can also be used to diagnose acute infection by detection of seroconversion in paired samples [8]. Virus neutralization (VN): is the reference test for antibody detection. VN is sensitive and specific, but cell culture dependent and labour demanding and will typically take 5-6 days to perform. Thus, it is mostly used as a reference test for back-up and calibration purposes [8].
Antibody ELISA: For testing of large series of samples, enzyme-linked immunosorbent assays (ELISAs) have many advantages over VN. They are rapid, relatively inexpensive both to establish and run, and are suitable for automation. Two principally different ELISA formats are common in use for antibody detection: indirect or blocking (competitive) assays. In the indirect format, specific antibodies are trapped by immobilized viral antigen, and subsequently detected using enzyme conjugated [22]. Virus isolation (VI): in bovine cell cultures, followed by identification of the viral isolate by immunoperoxidase or immunofluorescence staining is considered the standard reference test for detection of infectious virus. Although VI demands time, resources, and skill, it is a reliable and widely used method. The presence of antibodies, or cell toxic substances, or both in the sample, however, can yield a false negative result. Furthermore, because BVDV is a common contaminant of biological by-products of bovine origin such as fetal calf serum (FCS) and, consequently, bovine cell cultures grown in FCS-supplemented media, the cell culture system must be monitored to ensure freedom from contamination with virus or antibodies [23].
Detection of antigen
Assays for detection of viral antigen rely on existing BVDVencoded antigens in the sample material, i.e., there is no amplification of the target, as is the case for VI and RNA detection protocols. This minimizes the risk of cross contamination of Samples, and flavors detection of PI over acutely infected animals [22]. Several formats of ELISAs are commercially available for detection of viral antigens. The basic principle consists of the use of virus-specific monoclonal antibodies to capture viral antigens, followed by detection of antigen-antibody complex with enzyme-conjugated antibodies. Antigen ELISAs are widely used for identification of PI animals and can be used for detection of viruses in serum, Buffy coat cells or skin biopsies (e.g., ear notch samples). Like VI, antigen ELISAs may yield false negative results if antibodies are present in the sample. This should be considered when testing young animals that might have persisting maternal antibodies [8].
Immunohistochemical detection of antigen in skin biopsies, particularly ear notch samples, is another method that is used for identification of PI animals [24]. By use of separate sets of primers and probes, BVDV-1 and -2 can also be discriminated between in the same assay [25]. Genotyping: The genetic typing of BVDV has most frequently been based on sequence comparison of partial sequences of the 5’ NCR, Npro or E2 regions [26]. Analysis of the 5’ NCR, a highly conserved region of the genome, has shown to be a reliable and reproducible method for genetic characterization of BVDV isolates even though the genetic resolution obtained is not as high as that obtained with more variable regions as Npro or E2 [27]. Furthermore, it is the target region for most PCR-based diagnostics, and as such a suitable target for direct sequencing from the PCR product [28].
Differential diagnosis of BVD
BVD is differentially diagnosed with several diseases like: Malignant catarrhal fever bilateral corneal opacity(edema) or uveitis, dermatitis are typical; Rinderpest- tiger or zebra striping lesion is prominent in large intestine on chronic case; Bluetongue- Hemorrhage on the base of pulmonary artery and aorta with large quantity of plasma like fluid in thorax and pericardial sac; Salmonellosis-no oral lesion, diarrhea but have is zoonotic bluereddish discoloration of extremities, cyanosis; Johne’s disease corrugated appearance of intestine & also has no oral lesion and bottle jaw are typical and prominent mucosal folds [21].
Treatment Reducing Clinical Effects
It is common for producers to treat BVD animals with antibiotics, thinking it will help the cattle “get over the hump” of whatever they are experiencing. In animals that are showing clinical effects of BVD, the only true treatment available is supportive. Optimizing the animal’s ability to neutralize the virus may consist of reducing fever, countering any nutritional or metabolic disorders, and providing basic husbandry practices that one would apply to a diseased animal. Use of antibiotics may be an option to prevent secondary diseases such as BRD and in a form may help reduce clinical effects of BVD. An animal with an acute form of BRD will cause the immune system to weaken, allowing a BVD virus to overcome the animal’s immune system, causing an acute form of BVD. The use of antibiotics has been proven to be effective at fighting respiratory infections in cattle. Broad spectrum agents such as Nuflor, Excenel, LA-200, and Micotil, can reduce the severity and length of a bacterial infection. In the likely case that an animal is fighting BRD along with BVD, by reducing the extent of the respiratory infection it will reduce the likelihood that the animal shows clinical signs of BVD [29,30].
No True Cure
Due to the nature of viral infections, there is no treatment to fully cure an animal of a viral infection. As we have discussed there is a limited amount of treatment that may be performed with BVD animals; the key lies in prevention of disease that are: All purchased animals should be tested for PI status and quarantined to give time for acute infections to clear; Any animal leaving the farm (shows, leased bulls, etc.) should be quarantined for 30 days before re-entering the herd. Purchased pregnant animals should not only be tested themselves for PI status, but also their calves should be tested at birth to make sure they are not a PI [31].
Prevention and control Prevention
The global nature of the disease, along with the ease of transfer between carriers, makes prevention of BVD a formidable challenge. The challenge of eradicating, or even just controlling BVD, can be seen by the state-wide and national attempts made by groups of producers to control the disease. When the time comes for a producer to implement a prevention program, one must keep in mind that the program must be specifically attuned to the type of operation (cow-calf, stocker, feedlot, dry lot dairy, pastured dairy cattle, dairy calf ranch, etc.). There is no “one size fits all” prevention program. It is crucial that the Producer design and continuously adapt a program that specifically fits their operation [22]. Animal Husbandry: The start of any effective prevention program begins with basic animal husbandry practices. Scoring of the body condition may be done with relative ease, especially with the help of a veterinarian and records should be maintained to compare past results. The importance of not underestimating the body condition of the animals is a crucial step in preventing BVD within a herd. Animals under nutritional deficiency may become immunocompromised, hindering the immune response time [32].
Passive Immunity: Adequate quantity and quality of colostrum within the desired 3-hour post parturition window. The wellknown benefits of adequate transfer of immunoglobulins from dam to offspring can be summed up simply by the formation of a competent and responsive immune system. The rewards of immunoglobulin absorption of offspring therefore are linked directly to the protection of clinical diseases including prevention of BVD [28,29]. Active Immunity and Vaccination: Although removal of a PI animal is the primary form of halting the shedding of BVD, it has been shown that transmission of the disease will be hindered by an adequate vaccination program [7]. Wide ranges of vaccine-induced neutralizing titers were formed in calves with a two-series BVD specific vaccine of either an inactivated or modified live vaccine [7]. Other important aspects of the study were that the study population was of colostrum-deprived calves; although the importance of colostrum and passive immunity should not be underestimated, this shows that regardless of degree of passive transfer, vaccines are still effective at stimulating titers in immunoglobulin naive calves [28,29]. It is apparent that vaccination and passive immunity both offer degrees of protection against BVD but, when coupled together; the combination conferred a potent efficacy against shielding viruses [28, 29].
Testing: Testing cattle for BVD is the most important aspect of prevention for the disease in white blood samples will be necessary to detect BVD in some PI animals. Different test methods used by laboratories, veterinarians and producers will be highlighted in the following discussion. Immunohistochemistry (IHC) is the most practical approach to testing a herd for BVD. Ear notch samples are the most common tissue sample used for this test. This requires little labor in obtaining samples from large numbers of cattle with the use of an instrument such as ear notch pliers and the samples are also suitable for transport. This test is very accurate at identifying PI animals but cannot provide accurate results for transient animals [33]. Also, Polymerase Chain Reaction (PCR) tests used to detect BVD have been shown to be timelier compared to virus isolation tests. Additionally, PCR has the potential to detect BVD in its antigen-antibody complex. Therefore, unlike IHC, this test can be performed on calves under 6 weeks of age, because it makes no difference if colostral antibodies are bound with the antigen [24].
Culling: Within any herd attempting to prevent BVD a strict “test and cull” policy must be practiced. Regardless of other factors including the worth of animals, market prices, labor involved, these animals must be removed from the herd. Every day a transient or PI animal is with its herdmates, the potential for the disease to spread will not decrease [34]. Closed Herd: Once the herd has been tested and is clean of BVD, the next challenge in preventing the disease is maintaining a closed herd. Simply put, any animal being introduced to the herd should be properly quarantined until tested as negative for BVD. At this time, it is then safe for the animal to be turned out with other animals. Replacement heifers and bulls raised on a dairy or ranch are obviously exempt from this protocol, because of their origination from the herd. Maintaining a closed herd is a difficult task for producers to accomplish due to the transition of the beef and dairy industry to fewer and larger farms. This means that a larger number of animals are being moved in and out of farms in a shorter period [35].
Control
For long, attempts to control BVDV infections were limited to prophylactic treatment practices by using modified live vaccine and killed virus vaccine, implemented primarily to reduce, or prevent clinical disease on a herd basis. Today, however, it is well established that the benefit of preventing clinical disease in transiently infected animals is negligible when considering the overall prevention and control of the disease [27].
Bovine Viral Diarrhea Infection in Ethiopia
In Africa, few studies conducted on seroprevalence of the disease have indicated that the prevalence’s were varied from 70–83% [36]. Although Seroepidemiology of BVD were reported in European and some African countries (OIE, 2008), there is no research or published information available as to these authors knowledge on the investigation of BVDV infection in Ethiopia. But there was one study had done to detect the presence of BVDV infection and to determine the Seroepidemiology and associated factors in three agroecological zones in Ethiopia such as Jimma, Southwest Shoa, and West Shoa where BVD is more prevalent where due to the following factors tropical climate and humidity of the area with bimodal heavy annual rainfall ranging and traditional mixed crop-livestock agriculture of areas makes the infection more prevalence on these areas as a study [37].
Economic Significance Of BVD
BVDV adversely affects both health and productivity. The losses due to infection are diarrhea, decreased milk production, reproductive disorders, increased occurrence of other diseases, and death. The losses from fetal infection include abortions; congenital defects; weak and abnormally small calves; unthrifty, persistently infected (PI) animals; and death among PI animals. [6]. As we have introduced, the effects of BVD are wide-ranging that vague and diverse clinical signs observed with the disease, it is very difficult to clinically diagnose BVD infected cattle. The difficulty of clinically diagnosing BVD positive cattle impedes the studying of these cattle in a clinical setting. Regardless, understanding the nature of viral infections in animals it should be noted that the side effects are not usually contained to one specific area of the body. So, a virus such as BVD has been noted to have potential complications with every body system in cattle. Although rare, BVD viruses have manifested in ways as rare as causing skeletal deformities to severe neurological damage [34- 46].
Conclusions and Recommendations
An understanding of the pathology and pathogenesis of BVDV infection will guide and optimize diagnostic approaches. As the age of the animals and stage of infection varies, different tests and testing protocols need to be utilized. Highly sophisticated, but relatively cheap diagnostic tests, such as a combination of RTPCR and Ab monitoring on pooled material allow classification of the BVDV infection status of individuals and herds. A molecular epidemiological approach may be used to trace routes of BVDV transmission, and to identify and prevent risky behaviour within a BVDV control programme.
A single introduction of BVD into a susceptible population may result in widespread infection, and an introduced strain may circulate for many years and remain genetically unaltered. The significance of a single animal being ill may be overlooked, as often occurs with animals that are persistently infected. It is important to realize that this single case could be an indicator of the potential for greater losses, due to the reproductive, productive, and other effects of the pestivirus. Awareness of the presence of pestivirus, followed by appropriate management strategies, will minimize losses. Finally, the immune suppression, Persistent infection, production loss, reproduction defect and neurological defects all these make BVD as one the most important economical disease in veterinary world.
Based on the above conclusion the following recommendations
are forwarded.
- It should be maintained on closed herd (not always practical).
- Don does not purchase replacements from auction.
- Quarantine new arrivals and show stock for 30 days and test new arrivals for BVD- PI.
- Test purchased pregnant animals and their calf at birth. Keep records (reproductions and health).
To Know more about Journal of Dairy & Veterinary Sciences
Click here: https://juniperpublishers.com/jdvs/index.php
To Know more about our Juniper Publishers
Click here: https://juniperpublishers.com/index.php




No comments:
Post a Comment