Cancer Therapy & Oncology - Juniper Publishers
Abstract
Prostate cancer is somewhat unusual when compared with other types of cancer. This is because many prostate tumors do not spread quickly to other parts of the body. Some prostate cancers grow very slowly and may not cause symptoms or problems for years or ever. Even when prostate cancer has spread to other parts of the body, it can be managed for a long time, allowing men even with advanced prostate cancer to live with good health and quality of life for many years. Vitamin D is a steroid hormone that is thought to play a role in the etiology and progression of prostate cancer. Hormone activity requires binding to the vitamin D receptor (VDR), which contains several genetic polymorphisms that have been associated with risk of prostate cancer.
Introduction
Lung cancer is one of the most prevalent cancers in humans. In 2018 alone, lung cancer took the lives of 1.6 million people worldwide [1]. There are two main types of lung cancer, non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC), with NSCLC comprising ~85% of the cases [2]. A histologic sub-category of NSCLC is lung adenocarcinoma, accounting for ~40% of all lung cancer cases. The other main histologic sub-category is squamous cell carcinoma. Intra-tumor heterogeneity as well as high mortality among patients with NSCLC emphasize the unmet need to identify reliable prognostic markers that are unique to each subtype. Further, despite recent advances in treatment options for NSCLC, such as targeting of driver mutations, immunotherapy, radiotherapy, and platinum-based chemotherapy [3], inherent or acquired resistance to treatment is still inevitable. Thus, how to overcome treatment resistance and identify molecular biomarkers that can be used to discriminate between responders and non-responders remains a major research focus.
Insufficient DNA damage response (DDR), especially against DNA double-strand breaks (DSBs), results in genetic instability that is a root cause of most cancers. Nevertheless, cancer cells are addicted to existing DDR pathways to maintain infinite replication, making these pathways an obvious central target in anticancer therapy [4] RING (Really Interesting New Gene) domain-containing E3 ubiquitin ligases are a large family of enzymes that associate with an E2 ubiquitin-conjugating enzyme to ubiquitinate their substrates. The RING E3 ubiquitin ligase RNF168 -dependent ubiquitination signaling plays an essential role in the DDR [5]. RNF168 localizes at DNA repair sites and promotes ubiquitination of key DDR proteins to drive DNA DSB repair [6]. Previous studies have shown that RNF168 is a factor in chemotherapy resistance in various cancers [7]. In esophageal cancer, over-expression of RNF168 has been found to correlate with poor prognosis [7]. RNF168 is understudied in NSCLC, and nothing is known concerning whether it may have a role in NSCLC subtypes as a potential prognostic biomarker.
Here we investigated the role of RNF168 as a potential prognostic marker in NSCLC using popular online databases such as CBioPortal, Oncomine, KMPlotter, ProteinAtlas, and CellMiner CDB. We found that high mRNA levels of RNF168 correlated with poor patient prognosis in lung adenocarcinoma. Furthermore, we found that this gene was overexpressed in NSCLC, in both adenocarcinoma and squamous cell carcinoma. Finally, we identified RNF168 to predict sensitivity to docetaxel in lung adenocarcinoma.
Results
RNF168 is over-expressed in non-small-cell lung cancer
We used CBioPortal, to study the genetic alteration of RNF168 in NSCLC. We found the gene to be altered 16.78% of the time in a total of 1,144 cases of NSCLC, with amplification being the most common alteration, present in 15.12% of cases (Figure 1A). We then delved into NSCLC subtypes and found RNF168 to be altered in 4.78% of adenocarcinoma cases (Figure 1B). RNF168 alteration was greatest in squamous cell carcinoma, with 46.07% of the cases showing gene amplification (Figure 1C). We used Oncomine to compare the expression of RNF168 in normal lung cells to that of lung adenocarcinoma cells and found greater expression in adenocarcinoma cells (Figure 1D). As expected, RNF168 mRNA expression was higher in squamous cell carcinoma than in normal pulmonary cells (Figure 1E). Thus, RNF168 overexpression was present in NSCLC subtypes.

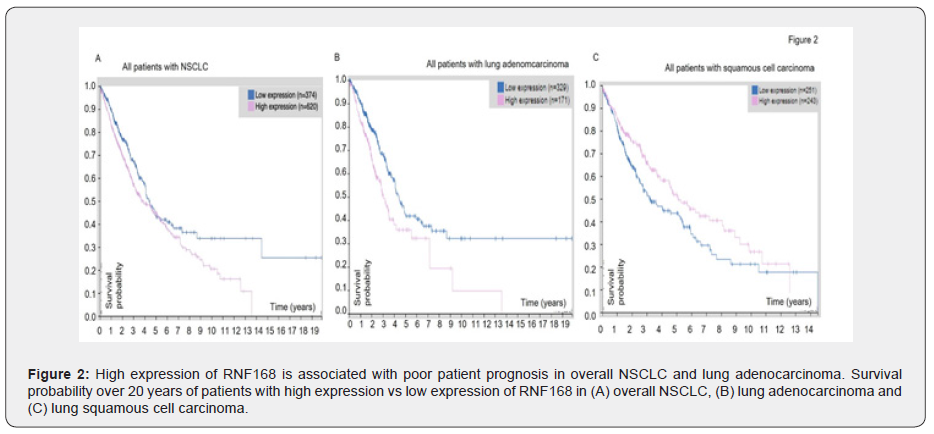
High expression of RNF168 is associated with lower patient survival in NSCLC overall and lung adenocarcinoma
Next, we looked at the patient prognosis using the Human Protein Atlas database to determine whether there was a correlation between gene expression and patient survival. We found that higher RNF168 expression was associated with significantly (P <0.007) decreased survival for all NSCLC patients, as shown in Figure 2A. This trend of poor patient prognosis when RNF168 level was high remained true for lung adenocarcinoma (P = 0.001) Moreover, the 5-year survival for patients with high levels of RNF168 was 36%, whereas of those who had low levels, 43% were alive at 5 years (Figure 2B). In squamous cell carcinoma, however, this trend was reversed. High levels of RNF168 consistently indicated better probability of survival (P = 0.02) (Figure 2C). Overall, our analysis of patient prognosis showed that high levels of RNF168 was detrimental to patients in overall NSCLC, and especially adenocarcinoma, whereas low levels of RNF168 indicated better patient prognosis for overall NSCLC and adenocarcinoma. In contrast, for squamous cell carcinoma, high expression of RNF168 was associated with better patient prognosis, and low expression of RNF168 was associated with poorer prognosis.
Docetaxel can be used to treat patients with high levels of RNF168
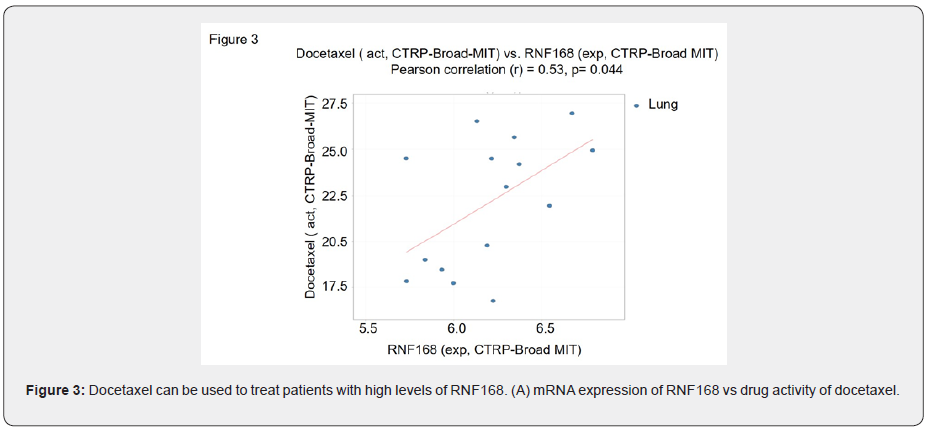
Our analysis of data from the Human Protein Atlas clearly showed that high expression of RNF168 suggested a poorer prognosis for patients with lung adenocarcinoma., Therefore, we used CellMiner CDB to look for drugs that exhibited a high efficacy in lung cancer cells with high RNF168 mRNA expression. We identified docetaxel as a drug that can potentially be used to treat patients with high levels of RNF168 (r = 0.53, r2 = 0.28, and P = 0.044; Figure 3).
Methods
CBioPortal
Through CBioPortal (https://www.cbioportal.org/), we identified RNF168 alteration frequencies in various types of lung cancer such as NSCLC, LUAD, and Lung Squamous Cell Carcinoma (LUSC). More specifically, we looked at the frequency of mutations, alterations, deep deletions, and multiple alterations in each histologic sub-category. We analyzed at the MSK, MSKCC, TracerX, University of Turin, and TCGA study datasets for NSCLC and LUAD to determine that RNF168 is over-expressed in NSCLC, LUSC, and LUAD.
Oncomine
Oncomine (https://www.oncomine.org) was used to
analyze the over-expression of RNF168 between different types of lung
cancer cells and normal cells, given by log2 median-centered intensity
and box and whisker plots indicating median intensities. The Hou Lung
dataset indicated RNF168 over-expression between patients with LUSC and
normal patients, while the Okayama Lung dataset showed the relationship
between RNF168 expression in LUAD patients and normal patients. Using
Oncomine, we could verify the over-expression of RNF168 in LUAD by
comparing the expression of the gene in LUAD with LUSC and normal lung
without cancer.
Human Protein Atlas
Using Protein Atlas (https://www.proteinatlas.org), we obtained prognostic data for RNF168 in overall NSCLC, as well as adenocarcinoma and squamous cell carcinoma. We used the prognostic information to analyze the effect of RNF168 on survival in NSCLC, LUAD, and LUSC, indicated by survival probability over 20 years. Patient data was taken from TCGA data sets.
CellMiner CDB
We used CellMiner CDB (https://discover.nci.nih.gov/cellminercdb/) to identify potential drugs that can target high RNF168 expression in adenocarcinoma. Univariate analyses allowed us to compare the patterns between mRNA expression (Z-score) and DNA copy number for RNF168 and drug activity for a variety of drugs. To determine the effectiveness of the identified drugs, the correlation between drug activity and RNF168 expression was analyzed using Pearson’s correlation, and we looked at cell lines from NCI-60, CCLE-Broad-MIT, GDSC-MGH-Sanger, and CTRP-Broad-MIT.
Discussion
We have found that high RNF168 levels were associated with a decreased probability of survival in patients with NSCLC patients, and particularly so for those with lung adenocarcinoma. Moreover, although high levels RNF168 indicates poorer survival for patients with adenocarcinoma, it has a better prognosis of patients with lung squamous cell carcinoma. Ultimately, we find that docetaxel is a potential treatment to patients with high RNF168 expression. Ubiquitin ligases (E3) have been of much interest to scientists because of their role in the degradation of proteins. RNF168 is no exception, and its potential to facilitate cancer progression as an oncogene has previously been identified. For example, RNF168 has been found to be over-expressed in esophageal cancer samples and correlated with poor patient prognosis. This study also showed that RNF168 promoted JAK-STAT signaling, which is involved in many different types of cancers [7]. Not only has RNF168 been shown to be involved in esophageal cancer, but many other cancers as well. Others have reported that overexpression of RNF168 can lead to mutations in the BRCA1 and BRCA2 genes, which are both tumor-suppressors [8]. BRCA mutation could lead to increased carcinogenicity and the investigators theorized that RNF168 can increase the risk of BRCA-mutated cancers. The impact of RNF168 on lung cancer has not been previously investigated. Our study adds to existing literature for RNF168 by giving further evidence of its potential as an oncogene. Our research also proof of yet another cancer that RNF168 contributes to, and thus underscores its role as a potential target in cancer. RNF168 is currently understudied, however, given its tumorigenic potential in adenocarcinoma, its role and regulation should be investigated in greater detail in the future studies.
Surprisingly, we found that high expression of RNF168 was associated with a better survival rate for squamous cell carcinoma patients. Most smokers who develop lung cancer suffer from squamous cell carcinoma, whereas adenocarcinoma is more likely to develop in non-smokers [9]. One of the primary causes of lung adenocarcinoma is genetic mutations, whereas squamous cell carcinoma is caused by the inhalation of carcinogens, damaging the cells that line the lungs [10]. The overexpression of RNF168 has previously been linked to the development of mutations, which could explain why high expression is detrimental in adenocarcinoma [7].
Conclusion
In conclusion, using data from public databases our study identifies a novel role of RNF168 as a potential biomarker in NSCLC [11-13]. While we find a positive correlation between overall survival in lung adenocarcinoma and negative correlation in case of lung squamous cell carcinoma, further research is needed, e.g. using RNF168 knockdown experiments in cell lines or mouse models to clarify a more deterministic role of RNF168 and mechanisms underlying the potential relationships between RNF168 expression and lung adenocarcinoma and between RNF168 and squamous cell carcinoma.
To Know more about Cancer Therapy & Oncology
Click here: https://juniperpublishers.com/index.php