Pharmacy & Pharmaceutical Sciences - Juniper Publishers
Abstract
A novel proprietary formulation was designed that consist of minerals (magnesium, zinc, copper, calcium, selenium, and iron), vitamins (ascorbic acid, pyridoxine HCl, alpha tocopherol, cyanocobalamin, and cholecalciferol), Panax ginseng extract, β-carotene, and cannabidiol isolate. The study objective was to evaluate the impact of Consciousness Energy Healing Treatment (the Trivedi Effect®) on a novel test formulation in male Sprague Dawley (SD) rats, fed with vitamin D3 deficiency diet (VDD) for immunomodulatory activity. The test formulation was divided into two parts. One part was denoted as the untreated test formulation without any Biofield Energy Treatment, while the other part was defined as the Biofield Energy Treated sample, which received the Biofield Energy Healing Treatment by renowned Biofield Energy Healer, Mr. Mahendra Kumar Trivedi. The level of total leukocytes count (TLC) were significantly increased by 23.1% and 17.44% in the Biofield Energy Treated test formulation (G5) and Biofield Energy Treated test formulation from day -15 (G7) groups, respectively compared with the disease control group (G2) induced by VDD. Moreover, the level of neutrophils was significantly increased by 71.68%, 92.92% (p≤0.01), 42.48%, and 49.56% in the G5, G7, Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15 (G8), and Biofield Energy Treatment per se animals plus untreated test formulation (G9) groups, respectively compared with the G2 group. Monocyte level was also increased by 23.53%, 17.65%, and 29.41% in the G5, Biofield Energy Treatment per se to animals from day -15 (G6), and G9 groups, respectively compared to the G2 group. Hepatic biomarker like alkaline phosphatase (ALP) was significantly reduced by 14.98% and 23.07% (p≤0.05) in the G7 and G9 groups, respectively as compared to the G2 group. Besides, cardiac biomarker like creatine kinase myocardium band (CK-MB) was also significantly (p≤0.05) reduced by 20.99% in the G6 group compared with the G2. Altogether, results suggested that the Biofield Treated test formulation and Biofield Energy Treatment per se significantly increased immune-related parameters, which might be beneficial for the management of immune-compromised patients as well as to boost-up the immunity in healthy peoples. The results showed a significant slowdown of disease progression and all other disease-related complications/symptoms in the preventive Biofield Energy Treatment group per se and the Biofield Energy Treated Test formulation groups (viz. G6, G7, G8, and G9) as compared to the disease control group.
Keywords: Biofield treatment; Immunomodulation; The Trivedi effect®; Hematology; Biochemistry; Vitamin D3 deficiency diet; Calcitriol
Abbreviations: CAM: Complementary and Alternative Medicine; IBS: Irritable Bowel Syndrome; QoL: Quality of Life; TLC: Total Leukocytes Count; SGOT: Serum Glutamate Oxaloacetate Transaminase; SGPT: Serum Glutamate Pyruvate Transaminase; ALP: Alkaline Phosphatase; SEM: Standard Error of Mean; ANOVA: One-Way Analysis of Variance; CK-MB: Creatine Kinase Myocardium Band; DLC: Differential Leukocyte Counts; SD: Sprague Dawley; API: Active Pharmaceutical Ingredients
Introduction
Lack of vitamin D3 has been directly linked to various health problems like cognitive decline, osteoporosis, depression, diabetes, cardiovascular disease, hypertension, and cancer [1,2]. It is also very essential for bone health in children and adults. The processes by which intake of vitamin D3 like synthesis through skin via UV-rays and absorption from foods become less efficient with age [3]. That is why, hypovitaminosis of vitamin D3 is more common worldwide [4]. Based on this situation authors constructed the current research work to assess the effect ofBiofield Treatment on hematology and biochemical parameters with special reference to hepatic and cardiac biomarkers after induction of vitamin D3 deficiency diet (VDD) in male Sprague Dawley rats. The novel test formulation, which is a combination of different minerals (selenium, zinc, iron, calcium, copper, and magnesium), vitamins (cyanocobalamin, ascorbic acid, pyridoxine HCl, alpha tocopherol, and cholecalciferol), cannabidiol isolate and Panax ginseng extract. The active pharmaceutical ingredients (API) used in this test formulation already has been extensively applicable as nutraceutical supplement [5-8].
Biofield Therapy is one of the approaches of Complementary and Alternative Medicine (CAM) therapies. At present, it is considering as the first-line model for the management of numerous chronic, life-style oriented and metabolic disorders. According to National Health Interview Survey (NHIS) 2012, reported that most of the Americans used the dietary supplement as complementary health approaches than conventional medicine therapy. Apart from this, The National Center of Complementary and Integrative Health (NCCIH) has recognized and accepted the Biofield Therapy as a CAM health care approach in addition to other therapies, medicines and practices such as Tai Chi, Ayurvedic medicine, Qi Gong, deep breathing, Rolfing structural integration, yoga, chiropractic/osteopathic manipulation, natural products, massage, relaxation techniques, meditation, aromatherapy, progressive relaxation, hypnotherapy, Pilates, acupuncture, mindfulness, healing touch, naturopathy, special diets, homeopathy, acupressure, cranial sacral therapy traditional Chinese herbs and medicines, movement therapy, guided imagery, Reiki, essential oils, and applied prayer (as is common in other religions, like Judaism, Buddhism, Christianity, and Hinduism).
Human Biofield Energy has certain kind energy that can work effectively [9]. CAM therapies have been extensively used all over the world for the benefits aspect in the healthcare system [10]. Biofield Energy can be harnessed and transmitted by individuals into both living and non-living things via the process of unique thought transmission process. Biofield Energy Healing Treatment (the Trivedi Effect®) has been published in numerous peerreviewed science journals with significant outcomes in many scientific fields such as cancer research [11,12], microbiology and biotechnology [13-15], pharmaceutical science [16- 19], agricultural science [20-22], materials science [23-25], nutraceuticals [26,27], skin health [28,29], human health and wellness. In this context, authors have planned to investigate the impact of the Consciousness Energy Healing Treatment (the Trivedi Effect®) on the test formulation for immunomodulatory activity concerning hematology and biochemical parameters.
Materials and Methods
Chemicals and reagents
Pyridoxine hydrochloride (vitamin B6), calcitriol, zinc chloride, magnesium (II) gluconate, and β-carotene (retinol, Provit A) were purchased from TCI, Japan. Copper chloride, cyanocobalamin (vitamin B12), calcium chloride, vitamin E (Alpha-Tocopherol), cholecalciferol (vitamin D3), iron (II) sulfate, and sodium carboxymethyl cellulose (Na-CMC) were procured from Sigma- Aldrich, USA. Ascorbic acid (vitamin C) and sodium selenate were obtained from Alfa Aesar, India. Cannabidiol isolate and Panax ginseng extract were obtained from Panacea Phytoextracts, India and Standard Hemp Company, USA, respectively.
Maintenance of animal
Randomly breed male Sprague Dawley (SD) rats with body weight ranges from 200 to 300 gm were used in this study. The animals were purchased from M/s. Vivo Bio Tech, Hyderabad, India. Animals were randomly divided into nine groups based on their body weights consist of 6 animals of each group. They were kept individually in sterilized polypropylene cages with stainless steel top grill having provision for holding pellet feed and drinking water bottle fitted with stainless steel sipper tube. The animals were maintained as per standard protocol throughout the experiment.
Consciousness energy healing strategies
The test formulation was distributed into two parts. One part of each ingredient was considered as the untreated test formulation, where no Biofield Energy Treatment was provided. Another part of each ingredient was received Biofield Energy Treatment by Mr. Mahendra Kumar Trivedi (the Trivedi Effect®) for ~3 minutes under laboratory conditions. Besides, three group of animals were also received Biofield Energy Treatment under laboratory conditions for ~3 minutes. The blessing/treatment was given to the test items by his physical presence without touching in the laboratory of Dabur Research Foundation, near New Delhi, India. Similar way, the control samples were also treated by a “sham” healer for ~3 minutes under the same laboratory conditions. The “sham” healer did not know about the Biofield Treatment. After that, the Biofield Energy Treated and untreated test formulations were kept in the similar sealed condition and used as per the study plan. The Biofield Energy Treated animals were taken back to experimental room for further proceedings.
Experimental procedure
Seven days after acclimatization, animals were randomized and grouped based on the body weight. Dosing for groups G7 and G8 were initiated on day -15 and continued till end of the experiment. However, G1 to G6 and G9 groups were dosed from day 1 till the end of experiment. All the animals except G1 group received vitamin D3 deficient diet (VDD) daily to the end of the experiment. Three weeks after the initiation of induction of VDD, all the groups were dosed with respective formulations. At the end of 8th weeks after bleeding, blood from all the animals was collected from the retro-orbital plexus using capillary tube for hematology analysis. From one portion of blood, serum was isolated for the analysis of biochemical parameters. The selective vital organs were collected and weighed.
Assessment of hematology parameters
Hematological parameters such as total leukocyte count (TLC), and differential leukocyte counts (DLC) were analyzed using Hematology analyzer (Abbott Model-CD-3700) in blood samples [30]
Assessment of cardiac and hepatic enzymes
Creatine kinase myocardium band (CK-MB), alkaline phosphatase (ALP), serum glutamic oxaloacetic transaminase (SGOT), and serum glutamate-pyruvate transaminase (SGPT) were analyzed using serum by Biochemistry Analyzer, Spectra lab A– plus, Italy [31,32].
Sample preparation for commercial products
The samples of excipients like Avicel, Microcrystalline cellulose, Sodium CMC and pharmaceutical dosage forms like Ophthalmic suspension and Tablet are prepared as per the procedure mentioned under sample preparation for nasal spray suspension for the determination of Avicel content.
Determination of body weight, feed Intake, and organ weight parameters
All the experimental animals were daily analyzed for their change in body weight, feed intake, and organ weight parameters, which was calculated by weighing the daily feed supply and the left-over amount that evaluate the average daily feed intake. The average intake of feed was recorded in every three days interval throughout the experimental period. After terminal bleeding, the animals were sacrificed and the following organs such as liver, lungs, kidney, brain, heart, eyes, pancreas, spleen, thymus, adrenal gland, intestine, and reproductive organs, i.e., testis, prostate, epididymis, and vas deferens were collected. These organs were trimmed off any adherent tissue and fat, as appropriate and weighed. The organ to body weight ratio percentage was identified by comparing the weight of each organ with the final body weight of individual rat [33]. All the data were reported through the study treatment regimen. Relative organ weight was calculated as per Equation 1.
Clinical sign and symptoms
All the animals in different test groups were analyzed for various clinical sign and symptoms in accordance with in-house protocol. Abnormal behavior in animals was recorded with the time of onset and disappearance.
Statistical analysis
The data were represented as mean ± standard error of mean (SEM) and subjected to statistical analysis using Sigma-Plot statistical software (Version 11.0). For multiple comparison Oneway analysis of variance (ANOVA) followed by post-hoc analysis by Dunnett’s test and for between two groups comparison Student’s t-test was performed. The p≤0.05 was considered as statistically significant.
Results and Discussion
Hematology parameters
The experimental data of hematology parameters in various groups (G1 to G9) are summarized in Table 1. The results suggested an improved animal hematology profile as compared with the disease control group (G2). The tested hematology parameters like total leukocytes count (TLC) were significantly increased by 39.53% (p≤0.01), 16.67%, 23.1%, and 17.44% in the G3, G4, G5, and G7 groups, respectively as compared with the disease control group (G2) induced by vitamin D3 deficient diet. Similarly, the level of neutrophils was significantly increased by 110.62% (p ≤ 0.01), 86.73% (p ≤ 0.01), 71.68%, 92.92% (p ≤ 0.01), 42.48%, and 49.56% in the G3, G4, G5, G7, G8, and G9 groups, respectively as compared with the G2 group. Moreover, the level of lymphocytes was increased by 29.42% (p≤0.01), 1.53%, 15.14%, and 5.78% in the G3, G4, G5, and G7 groups as compared with the G2 group. Further, the level of eosinophils was significantly increased by 41.18%, 23.53%, 17.65%, and 29.41% in the G4, G5, G6, and G9 groups, respectively as compared with the G2 group. Monocyte level was also increased by 41.18%, 23.53%, 17.65%, and 29.41% in the G4, G5, G6, and G9 groups, respectively compared to the G2 group.
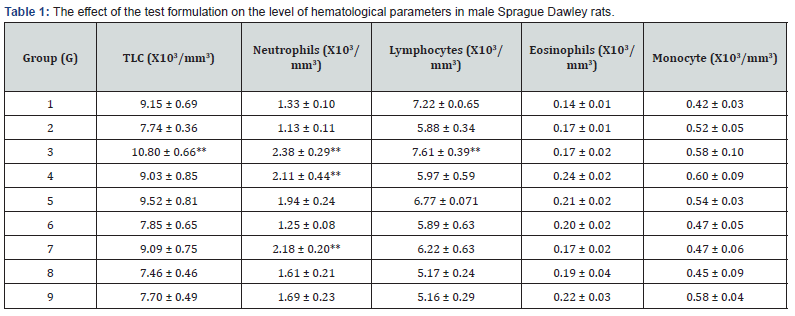
Altogether, study data suggested that the Biofield Treatment has the significant capacity to improve the blood immunity-related parameters. The altered hematology profile might be used in many acute infection, gout, rheumatoid arthritis, chronic inflammatory diseases, rheumatic fever, etc. However, minerals and vitamins play a vital role to control the hematology profile [34-36]. Increase the level of TLC and neutrophils count directly supports to the improvement of cell mediated immunity [37]. The study data concluded that the Biofield Energy Treated (the Trivedi Effect®) test formulation significantly improved the concentrations of TLC, lymphocytes, neutrophils, and monocytes in hematology profile assay, which suggest immunomodulatory potential of the test formulation with respect to altered hematological animal profile.
G: Group; G1: Normal control (0.5% CMC); G2: Disease control (VDD: Vitamin D3 deficient diet + 0.5% CMC); G3: Reference item (VDD + Calcitriol); G4: (VDD + Untreated test formulation); G5: (VDD + Biofield Energy Treated test formulation); G6: (VDD + Biofield Energy Treatment per se to animals from day -15; G7: (VDD + Biofield Energy Treated test formulation from day -15); G8: (VDD + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15), and G9: (VDD + Biofield Energy Treatment per se animals plus untreated test formulation). Values are presented as mean ± SEM (n=6). **p≤0.01 vs. G2.
Measurement of hepatic and cardiac biomarkers
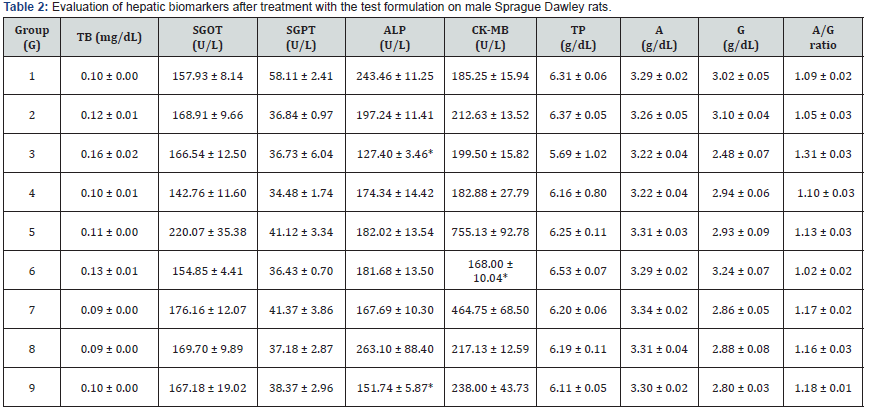
Hepatic and cardiac biochemical markers were tested for the test formulation and the results are tabulated in Table 2. The parameters used are serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), alkaline phosphatase (ALP) and cardiac enzyme creatine kinase myocardium band (CK-MB), and other biomarkers such as, total bilirubin, albumin, and globulin of different groups (G1 to G9) are summarized and compared with their respective controls. The level of ALP was also found to be significantly reduced by 35.41% (p≤0.05), 22.9%, 7.72%, 7.89%, 14.98%, and 23.07% (p≤0.05) in the G3, G4, G5, G6, G7, and G9 groups, respectively compared to the G2 group. Further, CK-MB was significantly reduced by 6.18%, 13.99%, and 20.99% (p≤0.05) in the G3, G4, and G6 groups, respectively compared with the G2.
G: Group; G1: Normal control (0.5% CMC); G2: Disease control (VDD: Vitamin D3 deficient diet + 0.5% CMC); G3: Reference item (VDD + Calcitriol); G4: (VDD + Untreated test formulation); G5: (VDD + Biofield Energy Treated test formulation); G6: (VDD + Biofield Energy Treatment per se to animals from day -15; G7: (VDD + Biofield Energy Treated test formulation from day -15); G8: (VDD + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15), and G9: (VDD + Biofield Energy Treatment per se animals plus untreated test formulation). Values are presented as mean ± SEM (n=6). *p≤0.05 vs. G2.
Liver toxicity was measured by the hepatic biomarkers, and any high alteration in these enzymes results in infection and liver damage [38]. The study data suggested that the Biofield Treated test formulation showed an improved liver and cardiac health as reflected by many improved levels of hepatic enzymes. Therefore, it can be concluded that the Trivedi Effect®-Biofield Energy Healing significantly improved the liver health and its immunity profile.
Estimation of animal weight parameters, feed intake, and relative organ weight
The test formulation in whole experimental study was calculated and defined with respect to weight parameters, feed intake, and relative organ weight. The results of animal tested organ weight parameters are summarized in the Table 3. The change in animal weights is reported as per the normal physiological process. Thus, the relative organ weight parameters did not show any significant change in the tested organ weight throughout the experiment suggested that the test formulation was found to be safe for the treatment. Organ to body weight ratio is the valuable index for any experimental test procedure with respect to the documentation of swelling, atrophy, or hypertrophy after exposure of test samples. Overall, the animal weight data, relative organ weight, and feed intake data suggested no significant abnormal change than the disease control group (G2). It suggests that Biofield Energy Treated test formulation and Biofield Energy Treatment per se were found as safe in all the tested animals.
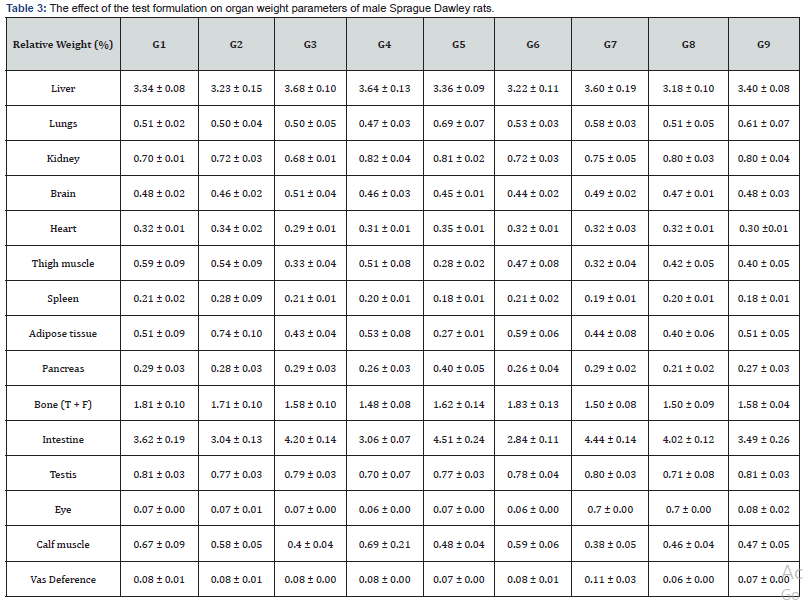
G: Group; G1: Normal control (0.5% CMC); G2: Disease control (VDD: Vitamin D3 deficient diet + 0.5% CMC); G3: Reference item (VDD + Calcitriol); G4: (VDD + Untreated test formulation); G5: (VDD + Biofield Energy Treated test formulation); G6: (VDD + Biofield Energy Treatment per se to animals from day -15; G7: (VDD + Biofield Energy Treated test formulation from day -15); G8: (VDD + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15), and G9: (VDD + Biofield Energy Treatment per se animals plus untreated test formulation). Values are presented as mean ± SEM (n=6).
this research plan, four groups were considered as preventive maintenance groups. These groups were G6 (Biofield Energy Treatment per se to animals at -15 days), G7 (Biofield Energy Treated test formulation from day -15), G8 (Biofield Energy Treatment per se to animals along with Biofield Treated test formulation from day -15), and G9 (Biofield treatment per se at -15 days to animals with untreated test formulation). Based on the overall data, it suggests that the Biofield Energy Healing Therapy was found to be most effective and beneficial to prevent and protect from the occurrence of any type of disease in the rat model. The data indicated that this therapy could act as a preventive maintenance therapy to prevent the occurrence of disease, slowdown the disease progression when disease-related complications are present which will ultimately improve the overall health and quality of life.
Conclusion
Blood profile data showed that the total leukocytes count (TLC) was significantly increased by 39.53% (p≤0.01) and 23.1% in the G3 and G5 groups, respectively compared to the disease control group (G2) induced by vitamin D3 deficient diet. Additionally, the level of neutrophils was increased by 110.62%, 86.73%, 71.68%, 92.92%, 42.48%, and 49.56% in the G3, G4, G5, G7, G8, and G9 groups, respectively as compared to the G2. Monocyte level was also increased by 41.18%, 23.53%, 17.65%, and 29.41% in the G4, G5, G6, and G9 groups, respectively compared to the G2 group. Besides, ALP level was reduced by 35.41%, 22.9%, 14.98%, and 23.07% in the G3, G4, G7, and G9 groups, respectively compared to the G2 group. Further, creatine kinase myocardium band (CKMB) was also reduced by 13.99% and 20.99% in the G4 and G6 groups, respectively compared with the G2. An experimental weight parameter such as body weight, organ weight, and feed intake data suggested normal changes, which suggest no toxicity profile of the test formulation. Thus, the present experiment suggested that the Trivedi Effect®-Biofield Energy Healing based novel test formulation have some impact on the immune system. The Biofield Energy Healing Treatment (the Trivedi Effect®) per se showed the best results with respect to different beneficial efficacy and biomarker parameters in the preventive maintenance group, G6, as compared to the other preventive maintenance groups (G7, G8, and G9) in the rat model study.
The Biofield Energy Healing Treatment also helped to slowdown the disease progression and disease-related complications impacting the overall animals’ health. These data suggested that Biofield Energy Treatment per se and Biofield Energy Treated Test formulation in combination would be the best treatment strategy to prevent and protect from the occurrence of any type of disease. Therefore, the Biofield Energy Healing Treatment (the Trivedi Effect®) per se might be an effective in healthy humans, when used as a preventive maintenance therapy to sustain good health, to boost overall health, promote healthy aging and increase quality of life.
In the presence of disease, the Biofield Energy therapy might reduce the severity of any acute/chronic disease (such as autoimmune related and inflammatory disorders) and / or slow the disease progression. Therefore, the Biofield Energy Treated test formulation and Biofield Energy Treatment per se may act as an effective anti-inflammatory and immunomodulatory product, and it can be used as a Complementary and Alternative Medicine (CAM) with a safe therapeutic index for various autoimmune disorders such as Systemic Lupus Erythematosus, Fibromyalgia, Addison Disease, Hashimoto Thyroiditis, Celiac Disease (glutensensitive enteropathy), Myasthenia Gravis, Pernicious Anemia, Aplastic Anemia, Dermatomyositis, Multiple Sclerosis, Chronic Fatigue Syndrome Graves’ Disease, Scleroderma, Psoriasis, Sjogren Syndrome, Crohn’s Disease, Vasculitis, Rheumatoid Arthritis, Reactive Arthritis, Type 1 Diabetes`, Vitiligo, and Alopecia Areata, as well as inflammatory disorders such as Irritable Bowel Syndrome (IBS), Asthma, Ulcerative Colitis, Alzheimer’s Disease, Parkinson’s Disease, Atherosclerosis, Dermatitis, Hepatitis, and Diverticulitis. Further, the Biofield Energy Healing Treated test formulation can also be used in the prevention of immunemediated tissue damage and can be used as a stress prevention and management which include overall health and improved Quality of Life (QoL).
To Know more about Pharmacy & Pharmaceutical Sciences
Click here: https://juniperpublishers.com/index.php





