Civil Engineering Research Journal
Introduction
General
Most of the synthetic organic chemicals and naturally occurring substances, enter the aquatic medium in several different ways and, according to their water solubility, can be transported and distributed in the water cycle. The effluents of urban wastewater treatment plants are among the major responsible for the release of this kind of contaminants into the environment. Although conventional biological processes are usually efficient for the degradation of pollutants occurring in wastewater, refractory compounds are not effectively removed. In such cases the use of Advanced Oxidation Processes (AOPs) may improve the overall removal efficiency of such compounds. In the Past two decades, advanced oxidation processes (AOPs) have been proven to be powerful and efficient treatment methods for degrading recalcitrant materials or mineralizing stable, inhibitory, or toxic contaminants. Advanced oxidation processes are those groups of technologies that lead to hydroxyl radical (_OH) generation as the primary oxidant (second highest powerful oxidant after fluorine). Hydroxyl radicals are non-selective in nature and they can react without any other additives with a wide range of contaminants. These hydroxyl radicals attack organic molecules by either abstracting a hydrogen atom or adding a hydrogen atom to the double bonds. It makes new oxidized intermediates with lower molecular weight or carbon dioxide and water in case of complete mineralization.
Gasoline compounds
1. BTEX: Benzene Toluene Ethyl Benzene and Xylene
2. MTBE: Methyl Tert-Butyl Ether
3. TBA: Tertiary Butyl Alcohol
Advanced oxidation process
Advanced oxidation processes (AOPs) refer to a set of chemical treatment procedures designed to remove organic (and sometimes inorganic) materials in water and wastewater by oxidation through reactions with hydroxyl radicals. AOPs involve two stages of oxidation:
1) The formation of strong oxidants (e.g., hydroxyl radicals)
2) The reaction of these oxidants with organic contaminants in water
However, the term advanced oxidation processes refer specifically to processes in which oxidation of organic contaminants occurs primarily through reactions with hydroxyl radicals. In water treatment applications, AOPs usually refer to a specific subset of processes that involve O3, H2O2, and/or UV light. However, in this analysis, AOPs will be used to refer to a more general group of processes that also involve TiO2 catalysis, cavitation, E-beam irradiation, and Fenton’s reaction. All of these processes can produce hydroxyl radicals, which can react with and destroy a wide range of organic contaminants.
UV/H2O2 Process
AOPs based on the UV/H2O2 system have shown high efficiency in the degradation of several organic compounds of environmental relevance. The UV/H2O2 process has additional advantages in that there is no sludge production and high rates of chemical oxygen demand (COD) removal can also be achieved. The oxidation products are usually low molecular weight oxygenated compounds that are easily biodegradable or, in some instances, the organic compound reduces to carbon dioxide and water. In the UV–H2O2 process the photolysis of hydrogen peroxide generates an effective oxidizing species hydroxyl radical (-OH). The oxidation potential of hydroxyl radical is 2.8eV, which can completely destroy the pollutants present in wastewater or convert them into simple harmless compounds. Combined AOPs are more effective than any of the individual agents (eg: ozone, UV, hydrogen peroxide).
Need for the study
Gasoline compounds (BTEX, MTBE, TBA) are very difficult to treat, due to their resistance to biodegradability, stability to light, heat and oxidizing agents. AOPs have been developed to degrade the non-biodegradable contaminants of drinking water and industrial effluents into harmless species (CO2, H2O, etc.). Combined UV and hydrogen peroxide oxidation are one of the advanced oxidation processes which have been successfully applied to the treatment of various water pollutants. This process implies such simple reactions as UV photolysis of H2O2. It is characterized by the generation of a very powerful oxidizing species, well known as hydroxyl radicals, in relatively high steady-state concentrations. These reactive radicals can decompose and even mineralize the gasoline compounds with high efficiency.
Objectives of the study
study the performance of process on the degradation of Gasoline compounds (BTEX, MTBE & TBA) present in the wastewater. To determine the optimal operating parameters such as Hydrogen peroxide dosages, Gasoline compounds concentration, Hydraulic retention time for the effective degradation of gasoline compounds.
Materials and Methodology
Synthetic sample preparation
BTEX: A 5ml of Benzene, Toluene, Ethyl benzene, Xylene was taken and made into 5000ml with distilled water. This is kept as synthetic solution from which the required concentrations of sample are obtained.
MTBE: The stock solution from which the sample is to be obtained was prepared by the following procedure. 13.5ml of MTBE was taken and made into 100ml with distilled water. This is kept as stock solution from which the required concentrations of sample are obtained. Initially 125ml of stock solution was taken and made to 5000ml with distilled water and this sample was used for further trails. The samples were prepared based on knowledge acquired from the literature review.
TBA: The stock solution from which the sample is to be obtained was prepared by the following procedure. 13.5ml of MTBE was taken and made into 100ml with distilled water. This is kept as stock solution from which the required concentrations of sample are obtained. Initially 125ml of stock solution was taken and made to 5000ml with distilled water and this sample was used for further trails. The samples were prepared based on knowledge acquired from the literature review.
Experimental procedure
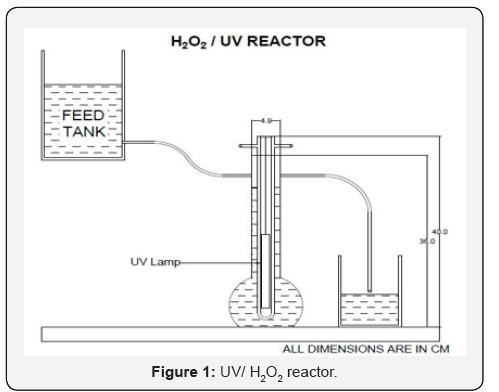
The reactor setup consists of a round bottom glass vessel 1.1 liters with inlet and outlet. UV lamp of 11W is inserted inside the glass vessel. The experiment is conducted for the gasoline concentrations by adding various hydrogen peroxide dosages 0.5ml, 1ml, 2ml, 5ml, etc. In the feed tank hydrogen peroxide dosages is added to produce powerful hydroxyl radicals. The BTEX, MTBE & TBA solutions are continuously flow through the reactor at a flow rate of about 9.12ml/min at the detention time of 120 minutes and it is irradiated with UV light inside the reactor. The reactor setup is kept inside the black box to avoid UV leakages. The treated samples are collected for every 10 minutes for 2 hours. Then the collected samples were tested for COD concentrations Figure 1.
Sample analysis
BTEX, MTBE, TBA samples were collected from the reactor at every 10 minutes interval for entire reaction period. The collected samples were tested for their COD values.
Results and Discussions
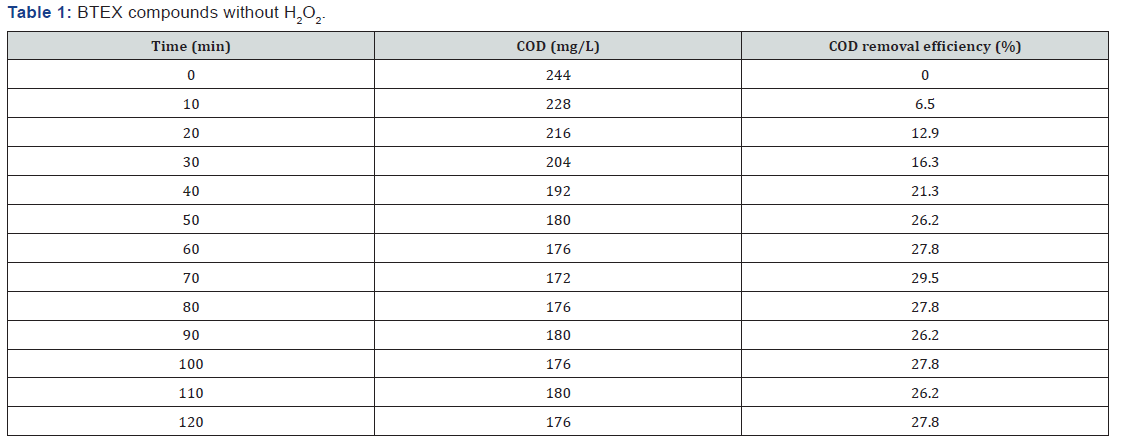
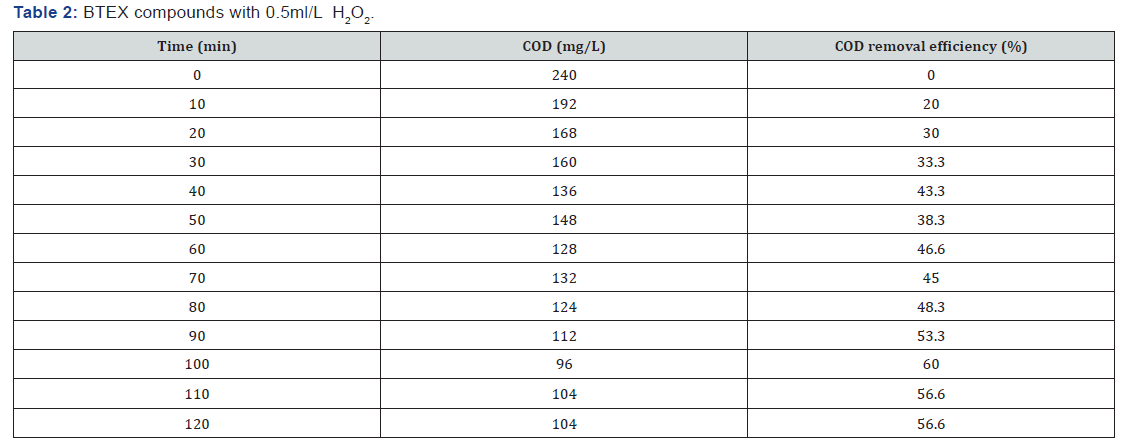
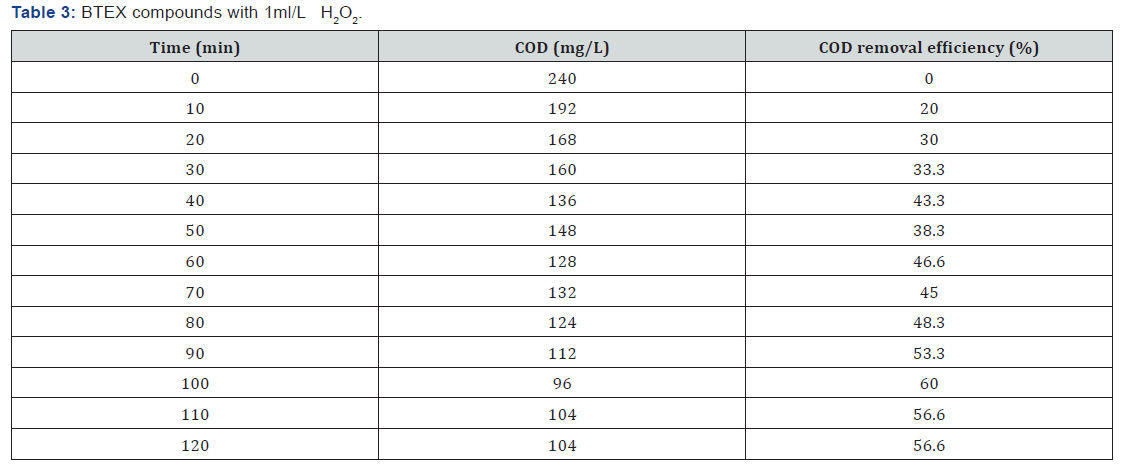
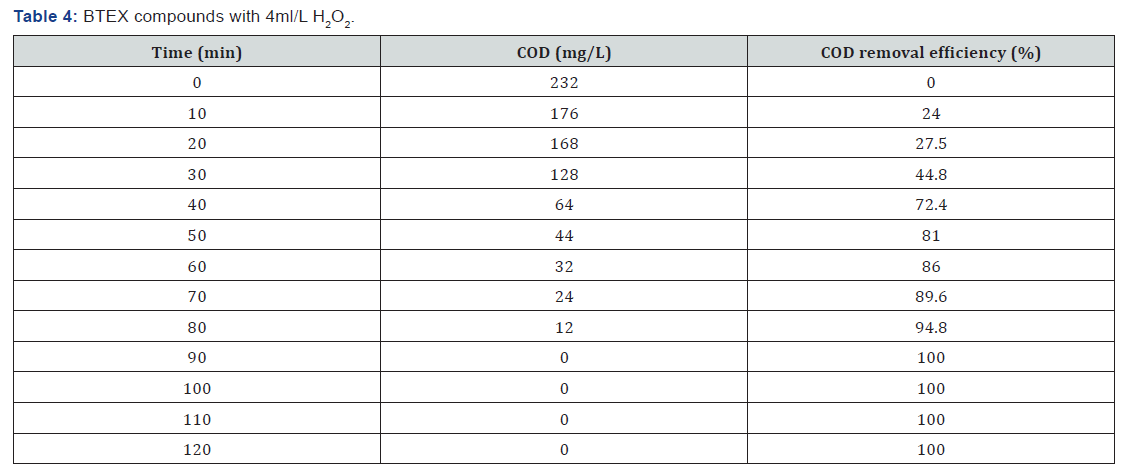
BTEX compounds cod reomval
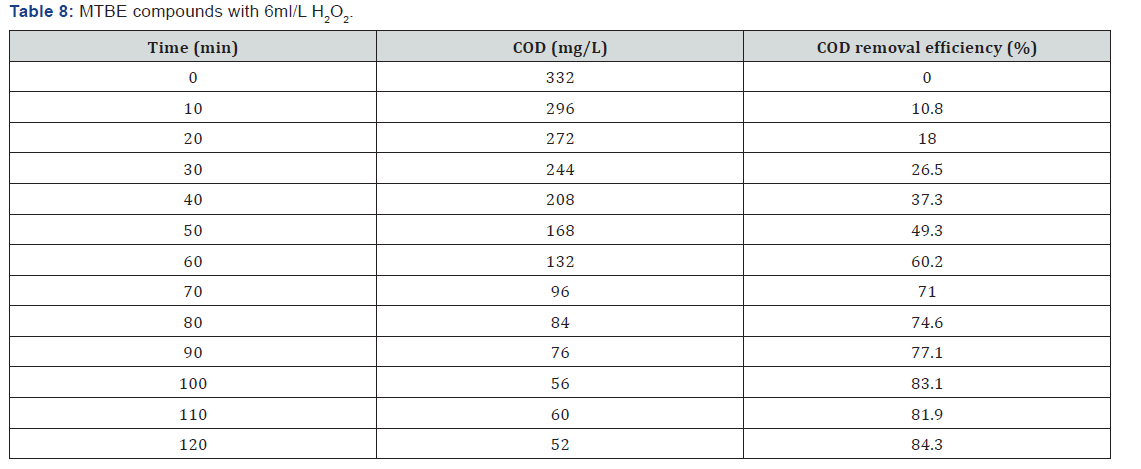
(Table 1-Table 8)



Conclusion
It can be concluded that in the wastewater treatment process of gasoline compounds presented sample the use of H2O2 in combination with UV resulted in an enhanced extent of COD removal. The COD removal efficiency of gasoline compounds decreased with increasing hydrogen peroxide dosages. When H2O2 concentration was increased the COD removal efficiency also increased. From the table 1,2,3,4 the optimized condition for H2O2/UV process was found at H2O2 dose of 4ml/L of wastewater, BTEX compounds concentrations maximum COD removal 100% achieved. From the table 5,6,7,8 the optimized condition for H2O2/UV process was found at H2O2 dose of 6ml/L of wastewater, BTEX compounds concentrations maximum COD removal 85.5% achieved. TBA compound will be treated enhanced results from the same process of other two compounds. It can be found that in the wastewater treatment process of Gasoline compounds (BTEX, MTBE, TBA) the use of H2O2 in combination with UV expected to result in an enhanced extent of COD removal.
To Know More About Civil Engineering Research Journal Please click on: https://juniperpublishers.com/cerj/index.php




No comments:
Post a Comment