Journal of Gynecology and Women’s Health
Diabetes mellitus (DM)
in pregnancy can be classified into either pre-existing diabetes or
gestational diabetes mellitus (GDM) [1]. Gestational diabetes is defined
as glucose intolerance with onset or first recognition during pregnancy
[2]. Several adverse outcomes have been associated with diabetes during
pregnancy and controlling blood glucose during pregnancy minimizes the
risk of complications.
Furthermore, intrapartum glycaemic control is
important for the foetus as factors such as foetal academia and
hypoglycaemia are strongly related to maternal hyperglycaemia during
labour. There is no clear recommendation regarding target blood glucose
during labour. The National Maternity Hospital (NMH) is a tertiary level
unit in Dublin, with more than 9000 births per year. A weekly
multidisciplinary clinic is provided by endocrinologists, obstetricians,
midwife diabetes specialists and dietitians. In this article we share
our experience in the management of blood glucose during labour for
patients attending the NMH with five different cases. These cases
involve the spectrum of diabetes in pregnancy which include: Type 1 DM
treated with insulin pump, Type 1DM on subcutaneous (SC) insulin, Type
2DM treated with subcutaneous insulin, GDM treated with Metformin and
GDM treated with SC insulin. A specific labour protocol was used for
each of the above patients which we believe contributed to good maternal
and foetal outcomes and good blood glucose control.
Keywords: Pregnancy; Diabetes; Insulin labour management
Cases
Case 1
33 year old female para 1+0 with a history of
gestational diabetes during her first pregnancy controlled with diet
only with no complications. GDM was diagnosed at 21 weeks of gestation
and treated with diet initially. At 22 weeks of gestation she required
metformin 500mg twice daily and was under regular follow up in the
maternity multidisciplinary diabetes clinic. Our patient had excellent
blood glucose control until the end of pregnancy with a HbA1c of 31
mmol/mol and a fructosamine level of 183-185.
μmol/L. Her foetal scan at 36 weeks showed
polyhydramnios and foetal abdominal circumference > 95th centile. At
39 weeks of gestation she went into labour and was started on protocol 1
for blood glucose control. She was prescribed to receive 2 units of
aspart (SC) if glucose ≥ 6mmol/L and 3 units of aspart if
≥ 8mmol/L. She underwent a normal vaginal delivery
and her labour lasted 4 hours and 34 minutes. Her blood glucose readings
during labour were 5.3, 4.4 and 5.6mmol/l. She had a healthy 3.8kg baby
boy with no neonatal hypoglycaemia. Post
labour her blood glucose was checked only before meals for 48 hours and
she was booked for an elective 2 hour 75g oral glucose tolerance test in
the 6-12 weeks post-delivery.
Case 2
2 year old female para 0 diagnosed with gestational
diabetes at 23 weeks of gestation. Initially started on diet control for
two weeks but her control was suboptimal. She was commenced on insulin
in the form of aspart and insulatard with regular follow up in the
multidisciplinary diabetes in pregnancy clinic. The insulin doses were
escalated to reach the target blood glucose and the patient required up
to 30 units of insulin daily in the third trimester of pregnancy. The
patient showed excellent blood glucose control, mostly on target, and
had a HbA1c of 35-37mmol/mol and a fructosamine level of 187-189 μmol/L.
Her foetal scan at 36 weeks of gestation was normal. At 37 weeks of
gestation our patient went into labour and was started on protocol 2 for
blood glucose control. She was prescribed one litre of Solution 18 with
20 mmol potassium chloride and 5 units of actrapid at an infusion rate
of 125ml/h. A supplementary SC sliding scale was also prescribed with 3
units of aspart if blood glucose ≥6mmol/L and 4 units of aspart if
≥8mmol/L. She underwent normal vaginal
delivery and her labour lasted 5 hours and 27 minutes. Her
blood sugars during labour were 4.9, 5.5 and 4.4mmol/l. She had
a healthy 2.6kg baby girl with no neonatal hypoglycaemia. Her
insulin was held post delivery and blood glucose before meals
was monitored for 48 hours. She was booked for an elective
2-hour 75g oral glucose tolerance test 6-12 weeks post delivery.
Case 3
29 year old female para 1+0 with a history of gestational
diabetes during her first pregnancy and postpartum type 2 diabetes
for 2 years duration. She was maintained on metformin
500mg twice daily with excellent control. She was evaluated by
our team at the maternity diabetes clinic at 5 weeks of gestation,
her initial HbA1c was 44mmol/mol and fructosamine level
was 208μmol/L. Her blood glucose readings were above target
and therefore her metformin was increased to 1000mg twice
daily and she was started on insulin in the form of aspart and
insulatard. She had regular follow up in the multidisciplinary diabetes
in pregnancy clinic. The insulin doses were escalated to
reach the target blood glucose and she required up to 47 units of
insulin daily. Our patient showed excellent blood glucose control
with the lowest HbA1c being 34 mmol/mol and a fructosamine
level of 187-194μmol/L. Her foetal scan at 37 weeks of gestation
was normal. At 38 weeks of gestation she went into labour
and was started on protocol 2 for blood glucose control. She
was prescribed one litre of Solution 18 with 20mmol potassium
chloride and 8 units of actrapid at an infusion rate of 125ml/h. A
supplementary SC sliding scale was also prescribed with 3 units
of aspart if blood glucose ≥6mmol/l and 4 units of aspart if ≥8
mmol/L. She was delivered by caesarean section which lasted
35 minutes with a blood sugar of 4.9 mmol/l one hour prior to
surgery, 6.1mmol/l during and 4.8mmol/l post-delivery. She delivered
a healthy 2.8kg baby girl with no neonatal hypoglycaemia.
Her insulin was stopped post delivery and her blood glucose
was monitored for 48 hours. Her metformin dose was reduced to
500mg twice daily with a plan to follow her in a general diabetic
clinic.
Case 4
35 year old female with a history of type 1 diabetes for 13
years duration. She was para 2 with two previous caesarean sections.
She had uncontrolled diabetes pre pregnancy with a booking
HbA1c of 67mmol/L and fructosamine level of 348μmol/L .
Her pre-pregnancy diabetic regime was detemir 16 units daily
and aspart 6 units with breakfast and lunch and 8 units with her
evening meal. She was evaluated by our team at the maternity
diabetes clinic at 5 weeks gestation and her insulin dose was
adjusted according to her blood glucose readings on a regular
basis. Her cetemir dose at its highest was 20 units per day and
her aspart dose was escalated to a total of 48 units daily. This
resulted in significant improvement in her diabetes control with
her lowest HbA1c being 47mmol/L and her lowest fructosamine
level being 241μmol/L. Her foetal scan at 37 weeks of gestation
was normal. At 38 weeks of gestation she went into labour and
was started on protocol 2 for blood glucose control. She was prescribed
one litre of Solution 18 with 20mmol potassium chloride
and 10 units of actrapid at an infusion rate of 125ml/h. A supplementary
sliding scale was also prescribed with 3 units aspart if
blood glucose was ≥ 6mmol/L and 4 units of aspart if ≥ 8mmol/L.
She delivered via caesarean section which lasted one hour with a
blood glucose of 6.9mmol/l prior to surgery, 6.2 and 6.9mmol/l
during and 7.2mmol/l post-delivery. She had a healthy 3.9kg
baby boy with no neonatal hypoglycaemia. Her insulin was reduced
post labour to detemir 15 units and aspart 5 units with
each meal. It was arranged that she be followed in a general diabetes
clinic post delivery.
Case 5
39 year old female with type 1 diabetes for 24 years. Her diabetes
was complicated by proliferative diabetic retinopathy and
nephropathy. Our patient was commenced on continuous subcutaneous
insulin infusion (insulin pump) with aspart insulin three
years ago due to frequent hypoglycaemic episodes. This was her
first pregnancy, and she was seen in our maternity diabetes clinic
at 4 weeks of gestation. She had three basal rates per day with
a carbohydrate correction ratio with all meals and an insulin sensitivity
factor of 1:2. The patient required an average of 30 units
of insulin per day. Her initial HbA1c was 79mmol/L and her fructosamine
level was 325μmol/L. Her insulin dose was adjusted to
reach the target for blood glucose control, requiring up to 5 basal
rates of insulin and an average of 55 units of insulin per day. This
improved her HbA1c to 41mmol/L and her fructosamine level
to 240μmol/L. Her foetal scan at 34 weeks of gestation showed
an abdominal circumference > 95th percentile. At 38 weeks of
gestation she went into labour and was started on protocol 3
for blood glucose control. She was prescribed one litre of solution
18 with 20mmol potassium chloride at an infusion rate of
125ml/h and an insulin pump rate of 0.5 units/hour. A supplementary
sliding scale was also prescribed with 3 units aspart if
blood glucose ≥6mmol/L and 4 units of aspart if ≥8mmol/L. She
delivered via caesarean section which lasted 40 minutes with a
blood glucose of 4.4mmol/l prior to surgery, 6.2mmol/l during
and 7.8mmol/l post-delivery. She had a healthy 3.8kg baby girl
with no neonatal hypoglycaemia. Her insulin was reduced post
labour with regular follow up in a diabetic clinic.
Discussion
Diabetes mellitus (DM) in pregnancy can be categorized
into either pre-existing diabetes or gestational diabetes mellitus
(GDM) [1]. In both categories there is a higher risk of complication
to the mother and the foetus. Preeclampsia, macrosomia,
maternal and infant birth trauma, fatal hepatomegaly or cardiomegaly,
operative delivery, perinatal mortality amongst others
are all complications of hyperglycaemia during pregnancy [3].
Management of diabetes during pregnancy depends on the type
and severity of diabetes. Pregnant women with pre-existing type
1DM can either be treated with subcutaneous insulin or continues subcutaneous insulin infusion (CSII). Those with pre-existing
Type 2DM can either be managed with insulin, oral hypoglycaemic
agents or diet. GDM can also be managed with diet alone,
oral hypoglycaemic agents and/or insulin. The main goal with
any management plan is to achieve normoglycemia and to prevent
maternal and foetal complications.
The occurrence of foetal acidaemia and hypoglycaemia is
strongly associated with maternal hyperglycaemia during labour
due to foetal hyperinsulinemia [4]. The reduced calorie intake
and cessation of oral intake during the latent phase of labour and
the higher energy requirement during the active phase of labour
are both implicated in the lower insulin requirement during labour.
Fluid solutions containing dextrose can also be important
for optimal myometrial function during labour [5,6].
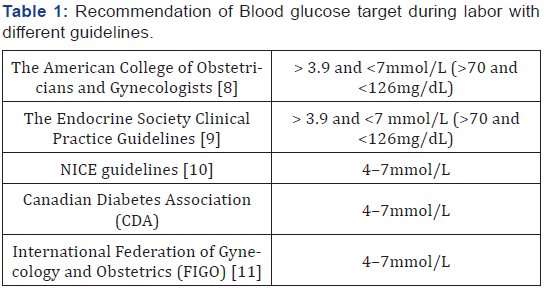
The metabolic changes during labour require close glucose
level monitoring. However, the ideal blood glucose target during
labour to prevent foetal complications is still unclear. The frequency
of monitoring of blood glucose during the intrapartum
period depends on the phase of labour. It is recommended to
monitor capillary blood glucose 2-4 hourly during the latent
phase of labour and 1-2 hourly during the active phase to achieve
good glycaemic control [4]. Several guidelines and recommendations
for target blood glucose have been summarised in Table
1. It’s important to note that a maternal blood glucose value of
more than 10mmol/L (180mg/dl) during labour has been proven
to be associated with a high risk of neonatal hypoglycaemia
[7].
The diabetic management plan during labour should be individualized
for each woman due to the differences in the type
and severity of diabetes, beta cell reserve and the severity of
insulin resistance. Unfortunately, a recommendation of optimal
approach to achieve normoglycemia intrapartum does not exist
due to the lack of well-designed, sufficiently powered, randomized
trials. Here we share our experience of managing blood
glucose levels during labour using fixed protocols. These protocols
are individualized according to the type of diabetes during
pregnancy, pre-delivery diabetic management and blood glucose
control [8-11].
Compliance with Ethical Standards
a) This paper was not funded
b) Author Sulaiman Haji Ali declares that he has no conflict
of interest
c) Author Recie Davern declares that she has no conflict of
interest
d) Author Mensud Hatunic declares that he has no conflict
of interest
e) Ethical approval: This article does not contain any studies
with human participants or animals performed by any of
the authors
f) Informed consent was obtained from all individual participants
included in the study (Protocol 1-3).
To Know More About Journal of Gynecology and Women Health Please click on: https://juniperpublishers.com/jgwh/index.php




No comments:
Post a Comment