Organic & Medicinal Chemistry - Juniper Publishers
Abstract
The several uses of zinc amalgam and conc. hydrochloric acid for the reduction of ketone and aldehyde (known as Clemmensen reduction) are described here. The reductions of 1,3-diketones, 1,4-diketones,
α,β-unsaturated ketones etc. afford many unexpected products.
Keywords:Clemmensen Reduction; Zinc Amalgam; Hydrochloric Acid; 1,3-Diketone
*Corresponding author: Ajoy K. Banerjee, Centro de Quimica, IVIC, Venezuela
1. Abstract
Introduction
The reduction of aldehyde or ketone 1 to the corresponding methylene compound 2 (Scheme 1) using zinc amalgam and concentrated hydrochloric acid (or gaseous hydrogen chloride) is known as Clemmensen reduction [1,2]. Generally, zinc amalgam and highly concentrated hydrochloric acid under reflux are employed to suppress the formation of by-products such as alcohols, dimerization products including pinacols and related compounds. Alcohol is not considered to be the intermediate since these intermediates are not reduced under Clemmensen reduction [3]. The original procedure is rather harsh and therefore the Clemmensen reduction of the acid-sensitive substrate and polyfunctional ketone seldom affords the desired product. Several modifications have been developed to improve the synthetic utility of the Clemmensen reduction. Generally, zinc amalgam and highly concentrated hydrochloric acid are employed to suppress the formation of by-products such as alcohols , dimerization products including pinacols and related compounds. However, among several modifications of this synthetic procedure, the method using zinc powder in acetic anhydride or ether saturated with hydrogen chloride is recommended [4-6] for its mild condition (0oC) and is valuable for reduction of aldehydes and ketones in molecules carrying such functional groups such as cyano, acetoxy, phenol ether and alkoxy carbonyl groups.
Mechanism
The reaction mechanism of Clemmensen reduction has not been clarified but it is well known that alcohol is not intermediate. As summarized, [1,6] in Scheme 2 the reaction is thought to occur on the zinc metal surface and involves protonation of the carbonyl function and a concomitant electron transfer process to give an organozinc intermediate (A). Furthermore, protonation of (A) followed by the abstraction of water and stepwise electron transfer yield a carbanion (B), which traps a proton and in the final stage the corresponding methylene group is formed by the exchange of zinc with another proton. A radical anion mechanism has been proposed for the Clemmensen reduction although the mechanism suggested in Scheme 2 is mostly accepted. It is recommended to go through the articles [7,8] to obtain more information’s on the mechanism of the Clemmensen reduction. The Clemmensen reduction may be promoted by ultrasound [9,10]. This would appear to be a good method to initiate heterogeneous Clemmensen reduction that takes place on the surface of the zinc metal. In one study the use of nonactivated zinc dust in acetic acid at room temperature with ultrasonic irradiation allowed the reduction of a less hindered ketone from a diketone substrate in good yield.
Applications
Several applications of Clemmensen reduction have been observed during the synthesis of organic compounds and natural products. The principal use of the Clemmensen reduction consists in the reduction of aldehydes and ketones to methylene compounds. The importance of the Clemmensen reduction in organic synthesis can be appreciated from the following examples.
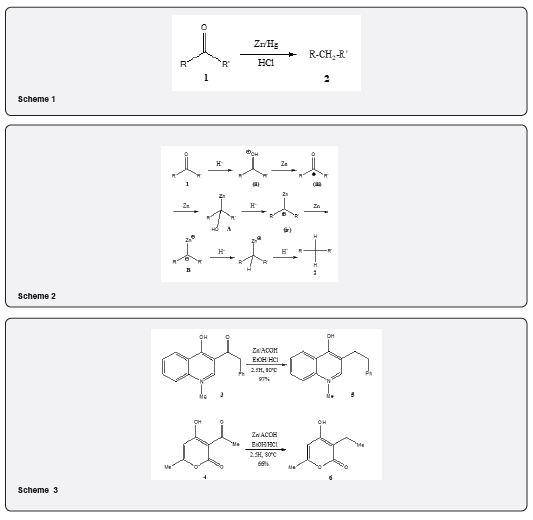
a) Synthesis of Quinolines and Pyran-2-ones: The Clemmensen reduction [11] has been utilized for the preparation of quinolines and pyran-2-ones. Thus 3-acyl-4-hydroxy-2(1H)-quinoline 3 and and 3-acyl-4-hydroxy-6-methylpyran-2-ones 4 have been reduced in good yield to obtain 3-alkyl-4-hydroxy-2(1H)-quinolines 5 and 3-alkyl-4-hydroxy-6-methylpyan-2-ones 6 respectively using zinc powder in acetic acid and hydrochloric acid (Scheme 3).
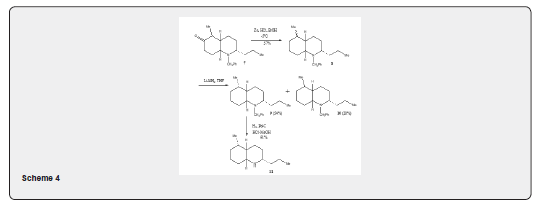
b) Synthesis of (-) of pumiliotoxin C: The clemmensen reduction has been utilized for the synthesis [12] of pumiliotoxin C 11 as depicted in Scheme 4. The cis-fused decahydro- quinoline 7 on being subjected to Clemmensen reduction with zinc, hydrochloric acid, ether at -5oC yields 2:1 epimeric mixture of the compound 8 which on reduction with lithium aluminium hydride followed by chromatographic purification yields 9 (54%) and 10 (28%). The compound 9 undergoes hydrogenolysis over Pd-C in methanolic hydrochloric acid leading hydrochloride salt of 5-epi-pumiliotoxin C 11. Many examples can be cited [13-15] to show that the importance of the Clemmensen reduction for the synthesis of terpenoid and steroidal compounds and also γ-lactones [16] which are important synthetic intermediates found in several natural products methods.
c) Some Newer Aspects of Clemmensen Reduction: It is necessary to mention that the Clemmensen reduction does not always provide the expected product. Some 1,3-diketones do not provide the expected product. In the case of derivatives carrying two carbonyl function stereochemically close to each other, Clemmensen reduction affords an interesting product distribution passing through characteristic intermediates [17].
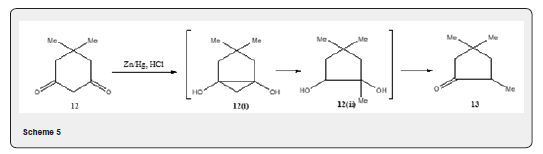
i. The diketone 12 gives 13 as major product under normal Clemmensen reduction condition [18]. The transformation passess through the intermediates 12i and 12ii (Scheme 5).
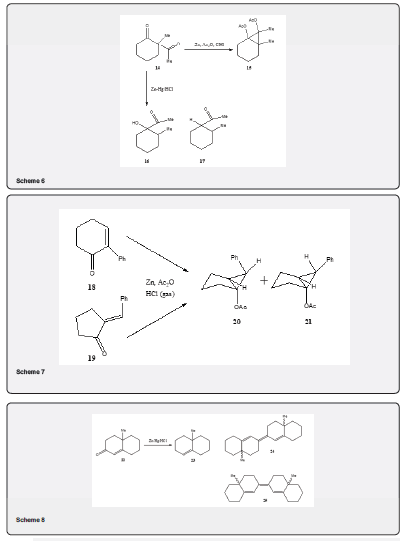
ii. It has been observed that the diketone 14 on treatment with zinc dust in acetic anhydride and hydrogen chloride yields the cyclopropane diacetate 15 In high yield. Similar result has been observed by repeating the experiment by electrolysis [19] (Scheme 6). The diketone 14 gives a mixture of rearranged products 16 and 17 under normal Clemmensen reduction using hydrochloric acid [20].
iii. With 1,4-diketones the distribution of the reduction products is dependent on the stereochemical situation of the carbonyl group [17]. In conjugated carbonyl system, the usual Clemmensen conditions may give rise to reduction and isomerization of double bond and dimerization. The reaction may include a cyclopropanol intermediate. The ketone 18 and the ketone 19 on treatment with zinc powder in acetic anhydride saturated with hydrogen chloride affords cyclopropanol acetates 20 and 21 in different ratio (Scheme 7) [21].
iv. The deoxygenation of the α,β-unsaturated ketone 22 under the Clemmensen condition leads to the formation of the olefin 23 (60%) along with the formations of the dimers 24 and 25 (30-40%) (Scheme 8) [22]. This paper describes the formations of dimers during the reduction of other unsaturated ketones.
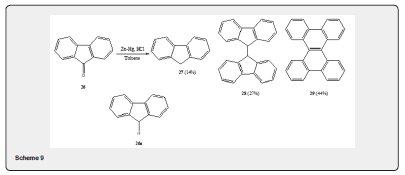
v. Talapatra and co-workers have observed [23] the formations of unexpected products during the deoxygenation of aromatic ketones by Clemmensen procedure. The reduction of 9H-fluoren-9-one 26 by the procedure of Clemmensen affords fluoriene 27 (14%), 9,9’-bifluorenyl 28 (27%) and dibenzo (g, p) chrysene 29 (44%). (Scheme 9). The reaction is probably proceeds via 26a. Similarly, the Clemmensen reduction of benzophenone and 9,10-anthraquinone yields dimerization products.
Conclusion and Comments
The above-mentioned discussion shows the importance of the Clemmensen reduction in the deoxygenation of carbonyl compounds. It is necessary to mention that the Clemmensen reduction does not always afford the expected product. Thus, the deoxygenation of cyclohexenone and cyclopenenone under the Clemmensen reduction with zinc, acetic anhydride in HCl (gas) leads the formation of dieastereomeric cyclopropanol acetates. A typical dimerization reaction in a conjugated system has been observed during the reduction of the octalone with zinc amalgam in concentrated hydrochloric acid under reflux. The reduction of aromatic ketones has produced bifluorenyl, tetraphenylethane and bianthrone [23]. The reduction of 4,4-diphenylcyclohex-2.en-1-one with zinc dust in hydrogen chloride in aprotic solvents affords different reduction products depending on the acid concentration [2]. The Clemmensen reduction has also been achieved by ultrasound [24].
To Know more about Organic & Medicinal Chemistry International Journal
Click here: https://juniperpublishers.com/omcij/index.php
To Know more about our Juniper Publishers
Click here: https://juniperpublishers.com/index.php





No comments:
Post a Comment