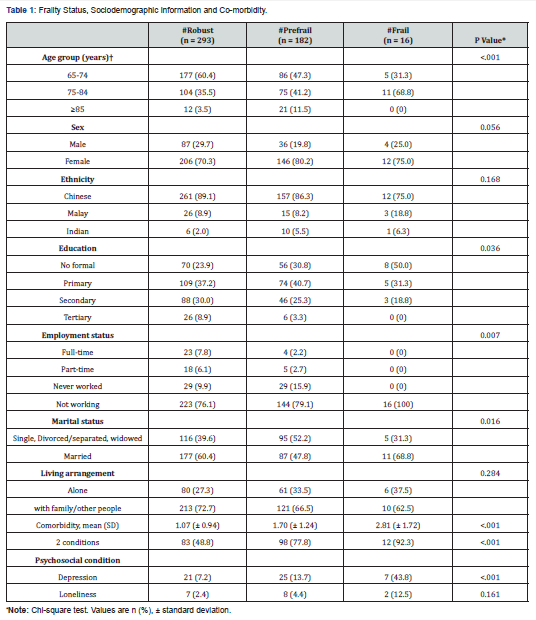
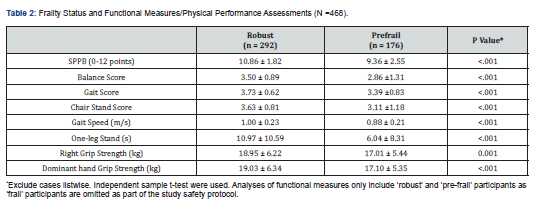
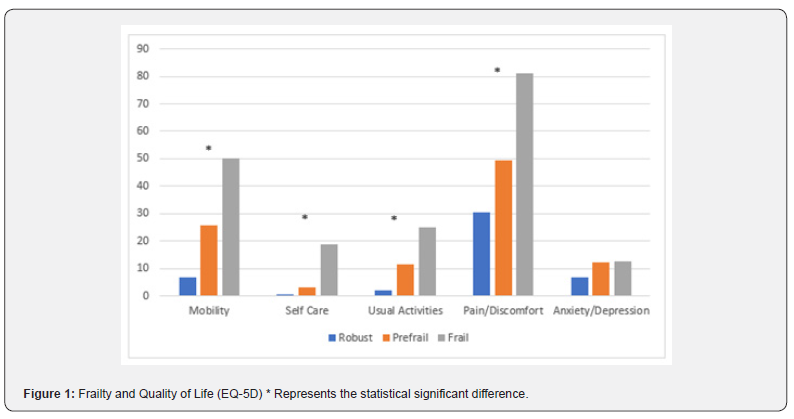
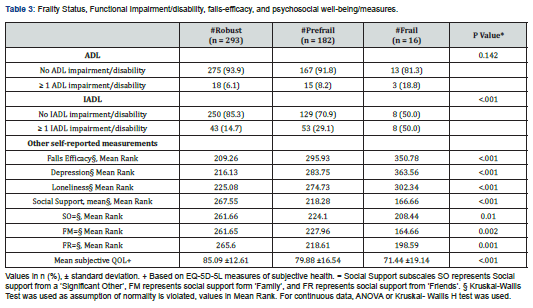
Drug Designing & Development - Juniper Publishers
Abstract
The aim of the present study is to optimize and develop tamoxifen citrate loaded PLGA (Poly lactide co glycolic acid) based nanoparticles for sustaining the action of tamoxifen citrate by controlling the release at a predetermined rate. Tamoxifen citrate is a non- steroidal anti- estrogenic drug used for breast cancer therapy and PLGA is a biodegradable co- polymer that can be given parenterally for its biocompatibility. The drug- excipients compatibility study, pre- formulation and post- formulation interaction and stability studies, preparation and characterization of the nanoparticles’ shape, surface, size, and size distribution have been performed. Tamoxifen citrate loaded PLGA based nanoparticles have been prepared by double emulsification and solvent evaporation technique with the variation of tamoxifen citrate- PLGA ratio, emulgent and stabilizer polyvinyl alcohol- water solutions’ concentrations and quantity, and speeds of homogenization to obtain the optimized nanoparticles with desired shape, surface properties, size and size distribution, drug loading efficiency, drug content and drug release profile. The characterization of particle size and shape have been performed by field emission scanning electron microscope and particles size distribution patterns and polydispersity indices have been studied by dynamic light scattering method using Zetasizer nano- series. The drug loading, drug content and in- vitro drug release studies of all the prepared batches have been performed by UV- spectroscopic analysis.
Keywords: Biodegradable; Biocompatibility; PLGA; Nanoparticles; Stability Studies; Homogenization; Antiestrogen; Estrogen; Breast Cancer; Cancer Chemotherapy; Drug Entrapment Efficiency
Introduction
Cancer is a major human health problem worldwide and it is the second leading cause of death in USA [1,2]. Breast cancer [3] is the most fatal disease especially for women world. Tamoxifen [4], a non- steroidal active antiestrogen and selective estrogen receptor modulator [5] (SERM), has been the clinical choice for the treatment of advanced or metastatic breast cancer for more than 30 years [2]. It has been used as adjuvant or additional therapy following primary treatment for early- stage breast cancer [6-8] and to treat post- menopausal breast cancer [9,10]. Microparticles [11,12] and nanoparticles [13] have been developed and utilized as anticancer drug delivery systems and are rapidly expanding area in the pharmaceutical fields. Nanoparticles [14,15] for their attractive properties occupy unique position in anticancer as well as other drugs delivery technology. The tremendous opportunities [1] exist for using nanoparticles [16] as controlled release drug delivery systems for cancer treatment [17,18]. PLGA is a USFDA approved copolymer for human use as surgical sutures, implantable devices and parenterally administrable drug delivery systems, owing to its biodegradability and biocompatibility [19]. Depending on the ratio of lactide to glycolide used for the polymerization, different forms of PLGA can be obtained and it undergoes hydrolysis in the body to produce the original monomers, lactic acid and glycolic acid. These two monomers under normal physiological conditions are by- products of various metabolic pathways in the body. Since the body effectively deals with the two monomers, there is very minimal systemic toxicity associated with using PLGA for drug delivery or biomaterial applications [20]. PLGA based nanoparticles [21] containing several types of drugs including anticancer drugs have been reported the uniqueness of this type of formulations [1]. Enhanced systemic stability of drugs or therapeutics, continuous and controlled drug release, reduced dosage and decrease in systemic side effects, reduced possibility of dose dumping, reduced frequency of administration and therefore increased patient compliance are some of the advantages of sustained release nanoparticles based on PLGA [19,22]. Therefore, an effort was made here to develop and evaluate (invitro) through optimization of various parameters biodegradable [23] PLGA (85:15) based nanoparticles for providing sustained release of tamoxifen citrate for the betterment in breast cancer therapy [24]. The development of drug delivery systems for cancer chemotherapy in the lowest dose of the drug (selective or specific) for providing the most patient compliance dosage form of available at minimum price and can work at the proper target site, is the most essential theme of the formulation scientists due to the high toxicity of the drugs which could lead to serious side effects as well as health hazards. Tamoxifen citrate is a costly drug, and the dose of tamoxifen citrate is 20- 40mg in a single dose or in 2 divided doses given orally in conventional tablet dosage forms. The optimum duration of treatment for breast cancer therapy using this drug is uncertain. The drug should be given at least 2 years and probably for 5 years or even lifelong left. For the longterm treatment policy, the development of PLGA based sustained release nanoparticles containing tamoxifen citrate is the interest of our research works and it is the need for the betterment of breast cancer therapy as well as to save the women from the fatal disease.
Materials
Tamoxifen citrate was obtained as gifts from Khandelwal Pharmaceutical (Mumbai, India). Biodegradable polymer Poly (D, L- lactide- co- glycolide)- 85:15 (PLGA) from Sigma- Aldrich Company (Bangalore, India), dichloromethane, dimethyl sulfoxide (DMSO) and methanol from Merk (Mumbai, India) were purchased. Polyvinyl alcohol (PVA) (M.W.-1,25,000) was purchased from S.D. Fine- Chem. Pvt. Ltd. (Mumbai, India) [25].
Methods
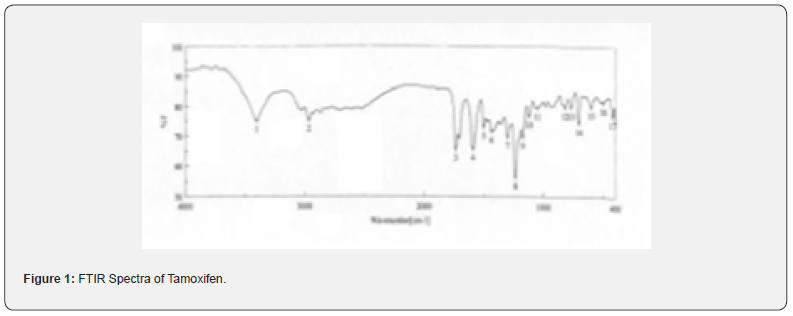
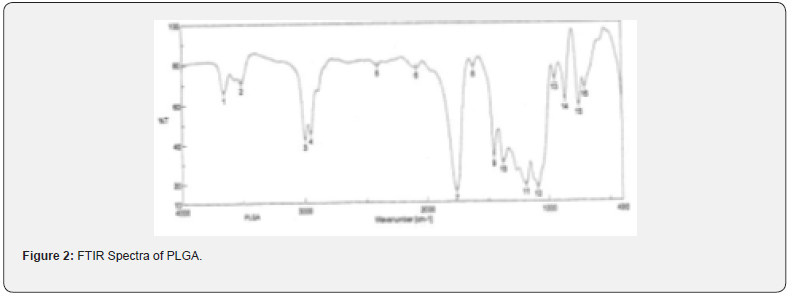
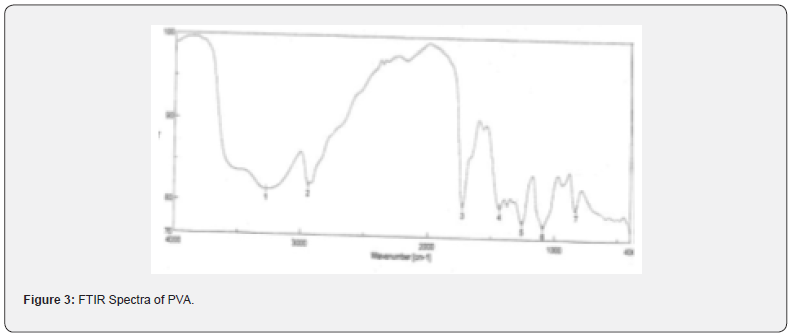
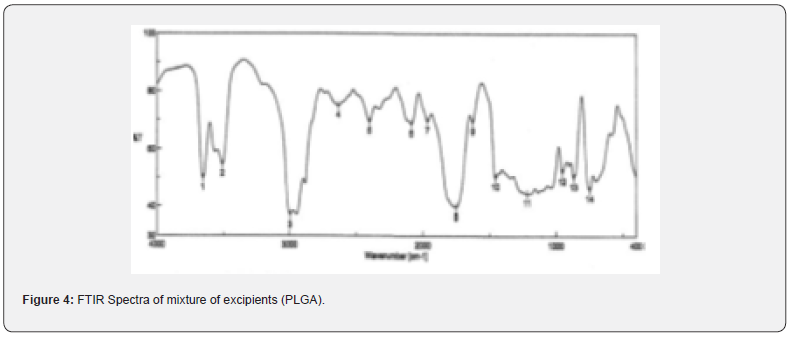
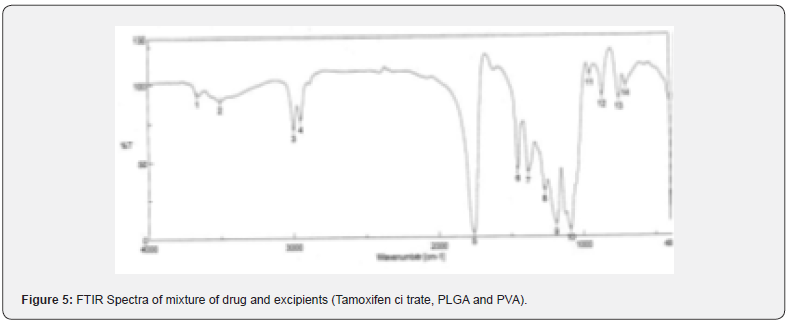
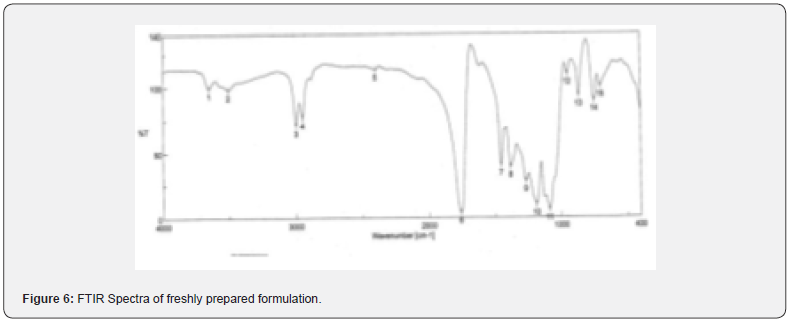
The pure drug tamoxifen citrate, PLGA- 85:15, PVA, a mixture of PLGA and PVA, and a mixture of tamoxifen citrate, PLGA and PVA were mixed separately with IR grade KBr in the ratio of 1:100 and corresponding pellets were prepared by applying 5.5 metric ton pressure with a hydraulic press. The pellets were scanned in an inert atmosphere over a wave number range of 4000- 400cm- 1 in Magna IR 750 series II, (Nicolet, USA) FTIR instrument [25] and the FTIR spectra were compared, no predominant interactions between drug and the excipients molecules were noticed. Only there is a mild interaction noticed between 1300 and 1200(cm- 1) wave number due to IR stretching vibration zone of functional group such as C- O (alcohol) and C- N [26,13]. There might be for the formation of weak physical bonds such as hydrogen bonding, bond due to Van der Waal’s forces or dipole moment between C- N present in the drug molecule and -OH present in the PLGA.
The data suggests that there is no chemical interaction that exists between the drug and the excipients since no shifting of peak was noticed as the shifting of peak is claimed as chemical interaction.
Nano- particles were prepared by double emulsification (w/o/w) and solvent evaporation technique. Desired amount of Tamoxifen citrate was taken in beaker with required quantity of DMSO/ methanol and 5%w/v PVA (M.W.- 125000) - water solutions were added to dissolve the drug with the help of sonicator (TRANS- O- SONIC, Mumbai), required amount of PLGA- 85:15 was weighed and taken in another beaker with desired quantity of dichloromethane to dissolve the PLGA. After dissolution of drug and PLGA, the required quantity of 2.5% w/v of PVA - water solution was added to drug- DMSO/methanol- PVA water solution slowly drop- wise to the oil phase i.e. PLGA - dichloromethane solution under continuous homogenization using high speed homogenizer at 6000 / 8000 / 10000 / 12000 / 13500 / 16000 r.p.m for 4 minutes, The first emulsion w/o was formed and it was added slowly to 75 ml of 1.5% w/v of PVA (M.W.- 125000, s.d.fine) solution under continuous homogenization at same speed 6000 / 8000 /10000 / 12000 /13500 / 16000 r.p.m respectively for 6 minutes and w/o/w emulsion was formed. Then the prepared emulsion was placed on magnetic stirrer for continuous stirring at room temperature for 12 hours for the evaporation of organic solvents DMSO / methanol and dichloromethane. The emulsion containing microparticles were filtered and washed with double distilled water by using cooling centrifuge (REMI) at 4ºC in speed 5000 or 14000 r.p.m. and kept the separated samples in freezer at - 20ºC (LG freeze) and dried in a freeze drier (Laboratory Freeze Drier, IIC Industrial Corporation, Kolkata) at - 42ºC [25]. The details of quantities of drug, polymer, stabilizer and solvents used for different batches of microparticles were given in Table 1.
Method for Optimization and Standardization of Formulations
Optimization and standardization were done to establish a standard formula, to optimize the speed of homogenization for preparation of formulations, speeds of centrifugation for separation of different particles size to obtain quality drug products with optimum and uniform range of particles size, drug content and drug entrapment efficiency in different variables by analyzing SEM and FESEM photographs, DLS zeta sizer particle size distribution analysis, drug loading efficiency and content estimation, and drug release profiles through UV visible spectrophotometric analysis of the drug product samples.
Method for Drug Content Studies
Nanoparticles (2mg) were dispersed in 1ml of 0.1M NaOH aqueous solution containing 5%w/v sodium dodecyl sulphate and in methanol- water (1%v/v) mixture for extraction of drug from nanoparticles. The extracts were filtered by centrifuge and 0.8 ml of supernatant was taken with the help of micropipette and absorbance was noted. The content of tamoxifen citrate and drug entrapment efficiency were calculated from standard curve using the following formula [25]. Percentage of Drug Content = (Weight of drug in the products /Total weight of the products) × 100 Percentage of Drug Entrapment Efficiency = (Weight of the drug in 1mg. product × Total products) / Total drug taken for formulation of a particular batch.
Method for Particle Size Distribution Analysis
The characterization of particle size and their distribution pattern were determined by Dynamic Light Scattering (DLS, Zetasizer nano ZS) and analyzed using DTS software (Malvern Instrument Limited, UK), Average particle size was calculated and expressed in nanometer [25].
Methods for Surface Morphology of Micro and Nanoparticles
The surface morphology of nanoparticles was investigated using Field Emission Scanning Electron Microscope (FESEM). Nanoparticles samples were mounted on the stubs using doublesided adhesive tapes. The stubs were then vacuum coated with platinum using JEOL JFC 1600 Autofine coater (JEOL, Tokyo, Japan). The nanoparticles were examined with FESEM, JEOL JSM 6700F (JEOL, Tokyo, Japan) [25].
Method for Drug and Drug Formulations Stability Studies
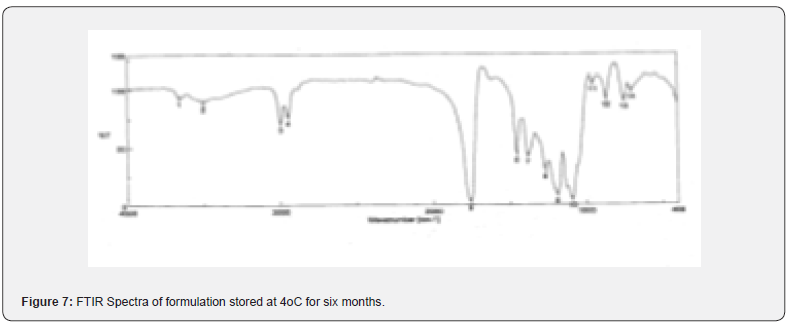
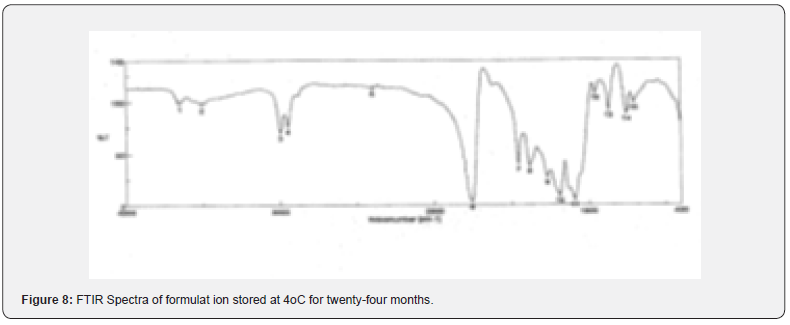
The pure drug tamoxifen citrate, a mixture of tamoxifen citrate, PLGA and PVA, a freshly prepared formulation, a formulation kept at 4oC for six months and a formulation kept at 4oC for twenty- four months were mixed separately with IR grade KBr in the ratio of 1:100 and corresponding pellets were prepared by applying 5.5 metric ton pressure with a hydraulic press. The pellets were scanned in an inert atmosphere over a wave number range of 4000- 400cm- 1 in Magna IR 750 series II, (Nicolet, USA) FTIR instrument [25] and a comparative study of FTIR spectra of different samples were done to analyze the stability of drug in the formulations.
Method for Drug Release Studies
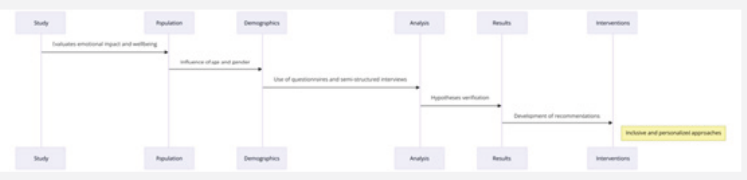
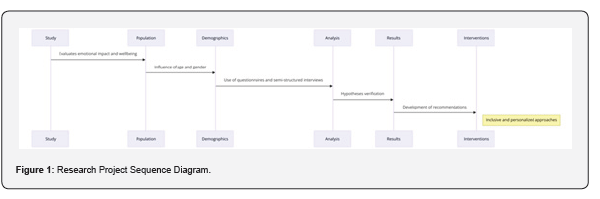
Nanoparticles (3mg) containing drug tamoxifen citrate were taken in 1ml phosphate buffer solution at pH- 7.4 as dissolution medium in 2ml Eppendorf tube and kept in incubator shaker at 37oC. Number of such tubes were kept for analysis of drug release at different time points. At a particular time point the tube intended for analysis at that time point was taken and the others remained in the incubator shaker to be analyzed at their respective time points. The tubes were centrifuged at 5000 rpm for 10 min, a measured quantity of supernatant was taken and analyzed spectrophotometrically at 238nm [25] (Figures 1-6) .
Results and Discussion
When the FTIR spectra of the different formulations Figures 7 and Figure 8 were compared with the spectra of individual drug, PLGA, PVA, mixture of drug, PLGA, PVA and freshly prepared formulation (Figures 1-6), no predominant chemical interactions between drug and the excipients molecules were noticed as all the characteristic peaks of the polymers were found to be present in the mixture of drug and excipients (Figure- 5), freshly prepared formulation (Figure 6), formulation stored at 4oC for six months (Figure 7) and formulation stored at 4oC for twenty four months or two years (Figure 8). A mild physical interaction was noticed between 1300 and 1200(cm- 1) wave number in the region of the IR stretching vibration zone of functional group such as CO (alcohol) and C- N for the formation of weak physical bonds such as hydrogen bonding, bond due to Van der Waal’s forces or dipole moment between C- N present in the drug molecule and -OH present in the PLGA. Since no shifting of peak was noticed as the shifting of peak is claimed as chemical interaction in Figure 7,8 [26,13], the chemical integrity of drug molecule should be maintained at least two years or formulations will be stable for two years in this particular storage condition.

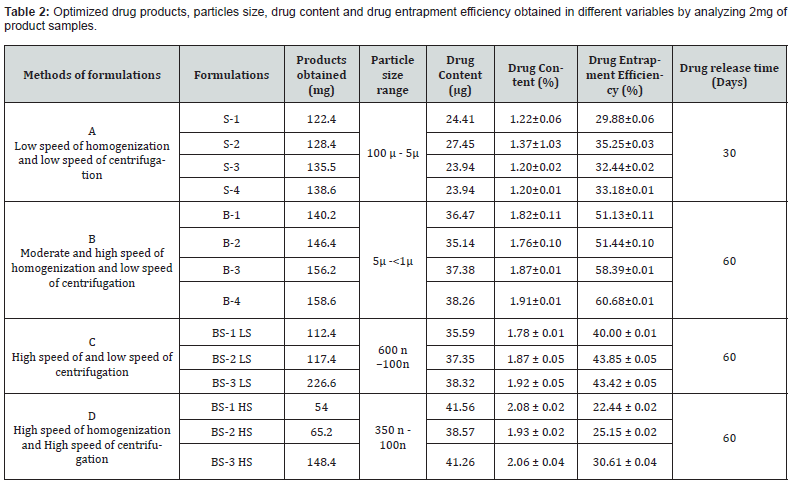
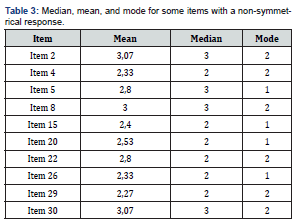
Drug Content and Drug Loading Percentage Efficiency
The drug loading values were expressed in terms of the quantity of drug entrapped in the formulations [2, 15] and the drug entrapment efficiency was associated with the percentage of total drug entrapped in a particular formulation [27]. The drug loading and the drug entrapment efficiency in the formulated particles can be enhanced by taking more drugs in preparatory steps and with the proper solvent [28]. The drug entrapment and content of the particulate drug devices gradually increased with the reduction of particles size. In this study results show the maximum drug content in the smallest nanoparticles formulation. The methanolic extraction method shows slightly better results due to the higher solubility of drug in methanol [27] causing better extraction of drug from formulation than SDS- NaOH solution (Table 2).
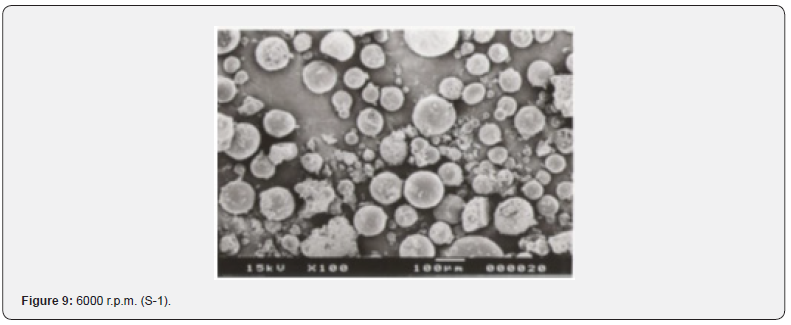
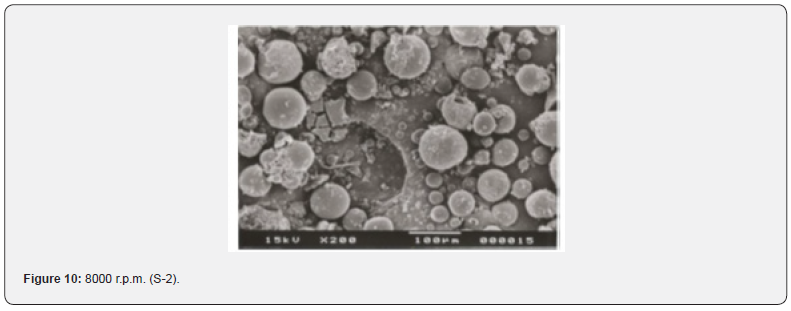
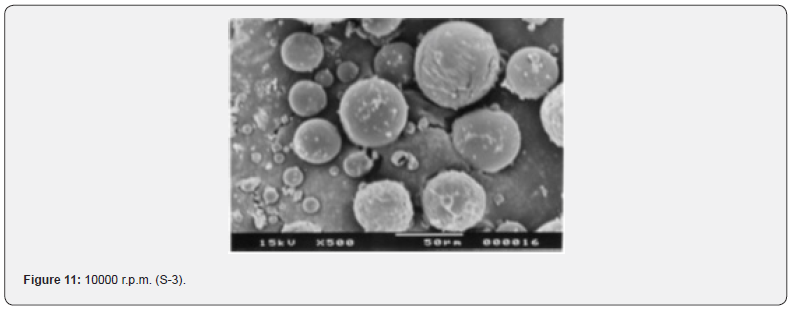
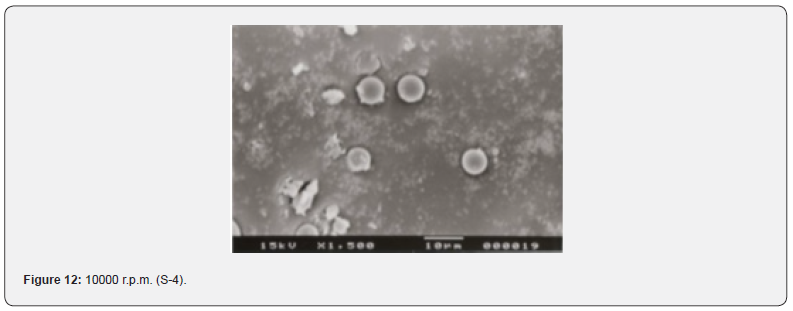
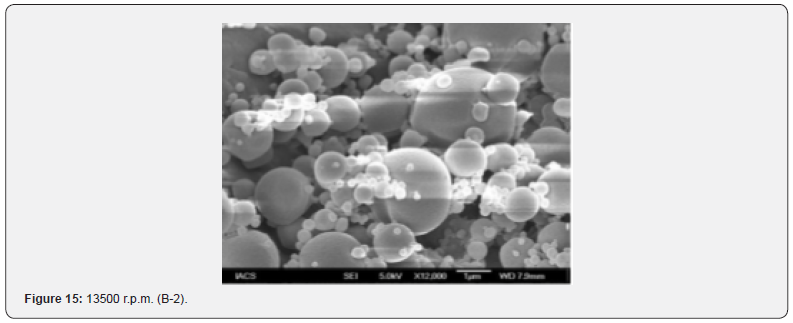
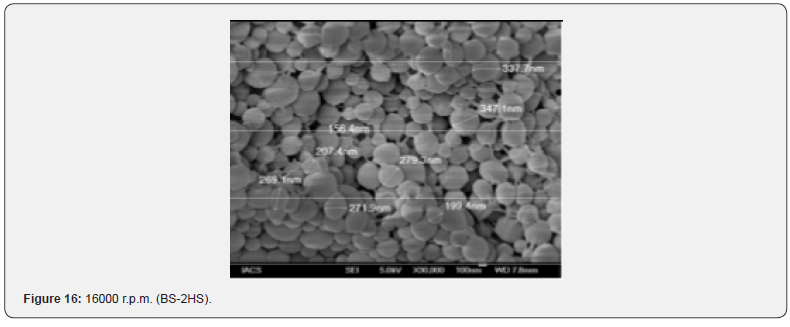
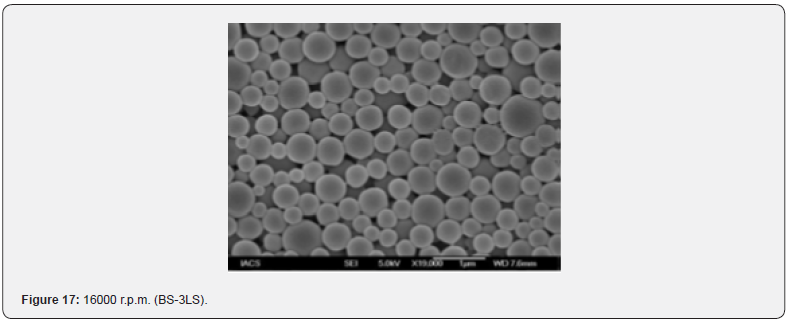
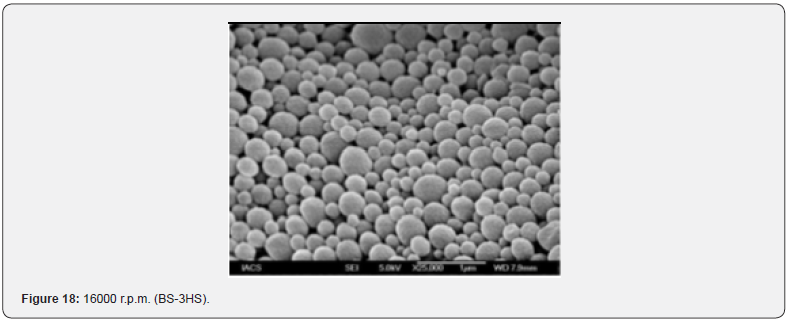
The Figures 9-14 show spherical microparticles of around 100 μ - 5μ range of particles, Figures 15 shows smooth surface micro and nanoparticles within the range of less than 5μ to 100 nm approximately, and Figures 16-18 show 100nm to 350nm in size range and few particles were of around 650nm. These formulations show the uniformity of the particle size due to high speeds of homogenization (16000 r.p.m) and centrifugation (14000 r.p.m) for preparations and separation of formulations respectively.


Particles Size Distribution Analysis and Polydispersity Indices (PDI)
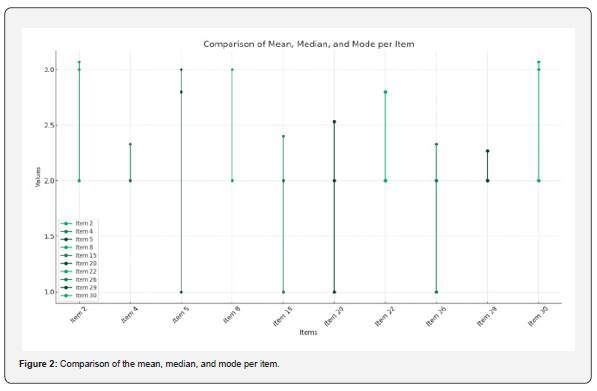
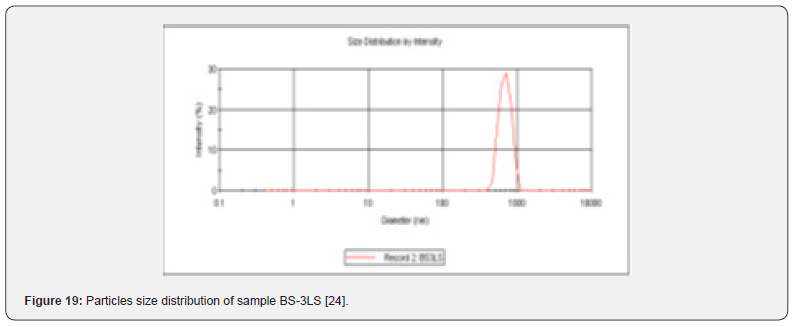
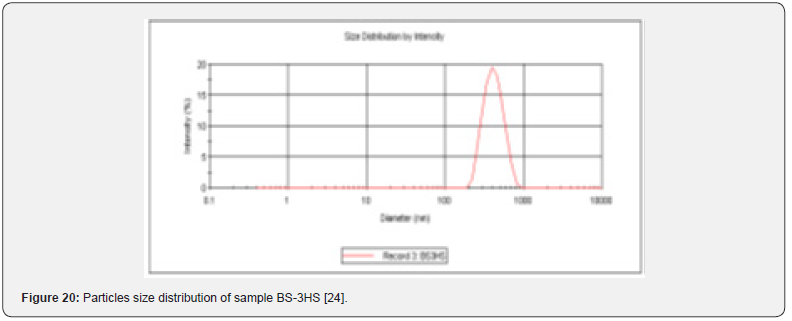
In the Figure 19 shows the particles size distribution varied from 400nm to1000 nm and the average particle size was about 650 nm with poly dispersity index (PDI) 0.04 of the sample formulation obtained from low- speed centrifugation (5000 r.p.m). The polydispersity indices suggest the particles were within nano range only and uniform monodisperse particle size distribution. The Figure 20 shows the particle size distribution pattern which ranged from 100nm to 800 nm and the average particle size was about 350 nm with poly dispersity index (PDI) 0.02 of the sample formulation obtained from high- speed centrifugation (14000 r.p.m).
In- Vitro Drug Release Profile of the PLGA Based Micro and Nanoparticles
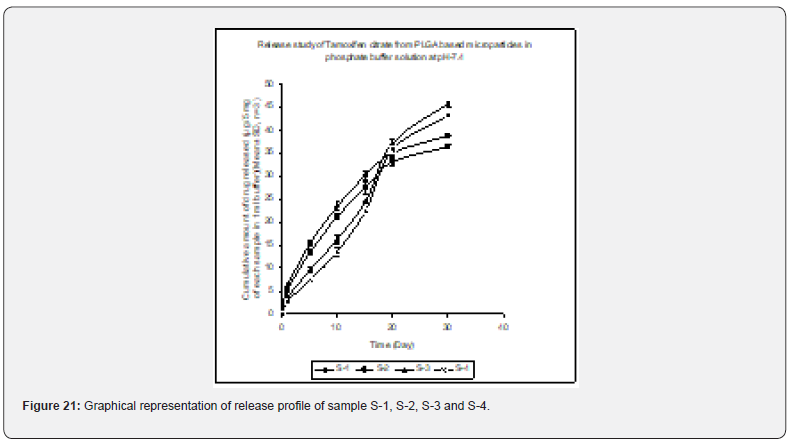
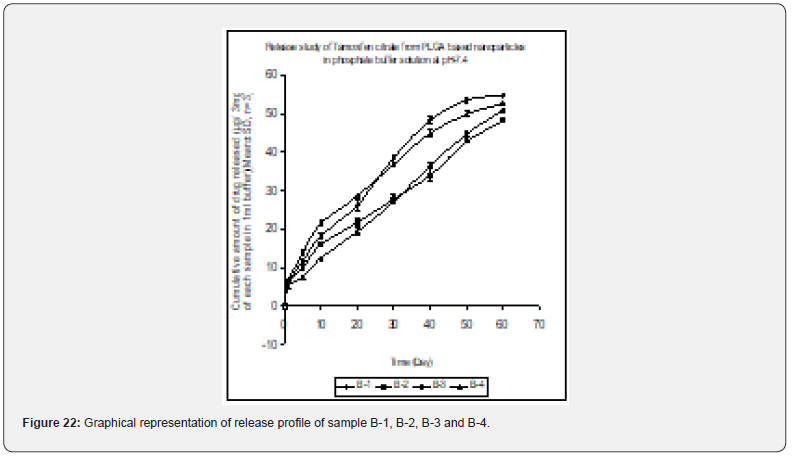
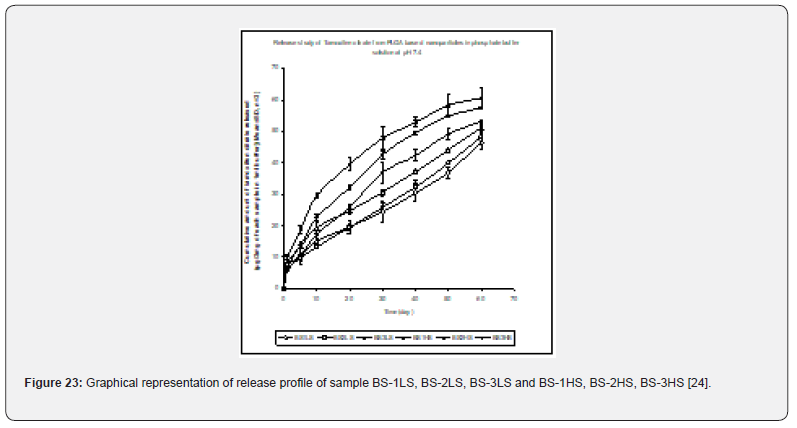
In- vitro drug release profile of Tamoxifen citrate from the PLGA based particulate drug formulations S- 1 to S- 4 was shown in Figure 21. The gradual increment of cumulative amount of drug release was seen and around 85% of the actual drug content in the formulations was released within 30 days. The results show the uniformity and faster drug release from formulations S- 1 to S- 4 as compared to the other experimental formulations. The results of in- vitro release profile of Tamoxifen citrate from formulations B- 1 to B- 4 (Figure 22) shows the gradual increment of cumulative amount of drug released and around 90% of the actual drug content in the formulations was released within 60 days. The results show the uniformity and slower drug release from formulations B- 1 to B- 4 as compared to the formulations (S- 1 to S- 4) and near about same as the formulations BS1LS to BS3HS. In- vitro release profile of Tamoxifen citrate from the PLGA based nanoparticle formulations BS- 1LS to BS- 3HS Figure 23 shows the gradual uniform release of Tamoxifen citrate and about 90% of drug was released within 60 days. The results show uniform and slower drug release from the experimental nanoparticles formulations. However, slowest and almost ‘zeroorder’ drug release was obtained in case of formulation BS- 1LS and faster and ‘first order’ kinetic pattern was observed in case of formulation BS- 1HS.
All the analytical data were analyzed at 238 nm for drug content study, drug entrapment efficiency study and in- vitro drug release study. The standard calibration curves show minor deviations which show favorable accuracy of our experiments.
Conclusion
The biodegradable polymer Poly (D,L- Lactide- co- Glycolide)/ PLGA- 85:15 based nanoparticles containing Tamoxifen citrate can be prepared by water- in- oil- in- water (w/o/w) emulsification and solvent evaporation method [29]. This method enables a high entrapment of hydrophobic (i.e Tamoxifen citrate) bioactive [30]. The particles size can be minimized by increasing the speeds of homogenizer up to a certain limit and uniform particle size distribution can be improved by the filtration or separation in different speeds of centrifugation. The particle size of the formulations was reduced with increasing the speeds of homogenizer. The uniform particle size was obtained by proper filtration by centrifugation with low poly dispersity index. The present study has been performed about the development and evaluation of nanoparticles containing anti- breast cancer drug with biodegradable polymeric devices which can be administered parenterally after reconstitution with sterile water for injection. This particular pharmaceutical product development was formulated with the biodegradable polymer [31] PLGA- 85:15 based nanoparticles with desired specific characters and successful entrapment, reasonable loading, controlled and prolonged release of tamoxifen citrate for the betterment breast cancer therapy. Thus, the mentioned nanoparticles formulation containing tamoxifen citrate could be beneficial system to deliver tamoxifen to breast cancer tissues through long term- controlled release for the betterment of the treatment via parenteral intramuscular or subcutaneous administration as a depot and it will be the potential alternative dosage form for the treatment of breast cancer. PLGA based nanoparticles containing tamoxifen citrate have been developed in the research laboratories. If this dosage form will be developed, established and available in the market, the patients as well as the society will be benefited due to the advantages of providing a long- term sustained release action after parenteral administration as a depot of the drug for the treatment of breast cancer. Therefore, we hope that in near future this dosage form will be reality for use economically and become a potential alternative to conventional controlled release dosage forms available in the market.
To Know more about Novel Approaches in Drug Designing & Development
To Know more about our Juniper Publishers