Pulmonary & Respiratory Sciences - Juniper Publishers
Abstract
Idiopathic Pulmonary Fibrosis (IPF) presents a complex challenge in respiratory medicine, characterized by insidious onset and relentless progression. This comprehensive analysis delves into predictive biomarkers across diverse domains to enhance early detection, precise patient stratification, and personalized therapeutic interventions. Blood biomarkers, including Krebs von den Lungen-6 (KL-6) and Surfactant Proteins A and D (SP-A and SP-D), offer diagnostic precision and prognostic insights, unraveling the dynamic interplay between immune responses and fibrotic activity. Genetic biomarkers highlight the role of genetic polymorphisms, shedding light on susceptibility and disease progression and paving the way for personalized therapeutic interventions. Imaging biomarkers, exemplified by High-Resolution Computed Tomography (HRCT), enable definitive diagnosis by unraveling distinct radiographic changes in IPF. Molecular biomarkers, such as microRNAs and cytokines, usher in a new era of molecular classification and prognostic assessments, promising tailored interventions based on molecular profiles. Cellular biomarkers, represented by circulating cells, provide non-invasive tools for disease monitoring and prognosis, showcasing the intricate interplay between immune responses and fibrosis. Collaboration across diverse research avenues has been employed in this synthesis to comprehend the complexities of IPF. Predictive biomarkers are beacons guiding the way toward earlier detection, precise stratification, and personalized therapeutic strategies, significantly improving respiratory health.
Keywords: Idiopathic pulmonary fibrosis; Predictive biomarkers; Blood biomarkers; Genetic biomarkers; Personalized therapeutics
Abbreviations: IPF: Idiopathic Pulmonary Fibrosis; KL-6: Krebs von den Lungen-6; SP-A: Surfactant Protein A; SP-D: Surfactant Protein D; ILD: Interstitial Lung Diseases; SNPs: Single Nucleotide Polymorphisms; DPP9: Dipeptidyl Peptidase 9; TGF-β1: Transforming Growth Factor-beta 1; CTGF: Connective Tissue Growth Factor; PDGF: Platelet-Derived Growth Factor; FGF: Fibroblast Growth Factor; VEGF: Vascular Endothelial Growth Factor; EGF: Epidermal Growth Factor; IGF: Insulin-Like Growth Factor; HRCT: High-Resolution Computed Tomography; UIP: Usual Interstitial Pneumonia; DLCO: Diffusing Capacity of Carbon Monoxide; PAH: Pulmonary Arterial Hypertension; miRNAs: MicroRNAs; AE: Acute Exacerbations; BAL: Bronchoalveolar Lavage; Th1/Th2: T Helper Cell Subsets 1 and 2; M1-M2: Macrophage Polarization States; CCL18: Chemokine Ligand 18; CHI3L1: Chitinase 3-Like 1; MMPs: Matrix Metalloproteinases; Tregs: Regulatory T Cells
Introduction
Idiopathic Pulmonary Fibrosis (IPF) presents a formidable challenge in respiratory medicine due to its insidious onset and relentless progression. Recognizing the critical need for early detection and precise patient stratification, this comprehensive analysis delves into the realm of predictive biomarkers in IPF [1]. Current diagnostic methods often capture the disease’s advanced stages, making early detection imperative [2]. This comprehensive analysis explores predictive biomarkers, aiming to unravel their potential to afford a swifter and more precise diagnosis and enable nuanced patient stratification based on distinct prognostic trajectories [3]. From the intricacies of molecular signatures detectable in blood to the nuances revealed by cutting-edge imaging technologies, each facet of predictive biomarkers in IPF will be meticulously examined [4]. The synthesis of these insights promises to revolutionize the diagnostic paradigm and open avenues for tailoring therapeutic approaches to individual patient profiles. To understand the complexities of IPF, this review seeks to pave the way for a future where early intervention and personalized care redefine the trajectory of this challenging pulmonary disorder.
Blood Biomarkers
Krebs von den Lungen-6 (KL-6)
The Krebs von den Lungen-6 (KL-6) antigen and surfactant proteins A and D (SP-A and SP-D) are the primary biomarkers linked to damage (or malfunction) of alveolar epithelial cells.
Serum KL-6 is more accurate than SP-A and SP-D in diagnosing interstitial lung illnesses (ILD and IPF linked to connective tissue diseases) [6]. KL-6 is a mucin-like glycoprotein expressed on the extracellular surface of bronchiolar epithelial cells and alveolar type II cells. It is a chemotactic factor to encourage lung fibroblast migration, proliferation, and survival. When it was initially studied as a serum tumor biomarker, patients with pulmonary fibrosis experienced a relatively high proportion of false positives.
A later study identified it as a biomarker for ILDs. Serum KL-6 has been linked to survival. Those with a serum KL-6 level >1000U/mL had a deteriorated survival compared to those with lower levels in a prospective trial involving 152 patients with idiopathic interstitial pneumonia and 67 individuals with interstitial lung disease associated with connective tissue disease. According to multivariate analysis, elevated blood KL-6 levels were independently linked to survival (HR 2.95, 95% CI 1.71-5.08, p=0.0001) [5]. Data from a study with a large Japanese sample were used to create (Figure 1) to show the different rates at which KL-6 tests positive based on a cut-off value of 500U/mL; data are from 225 patients with various lung diseases and 200 healthy individuals.
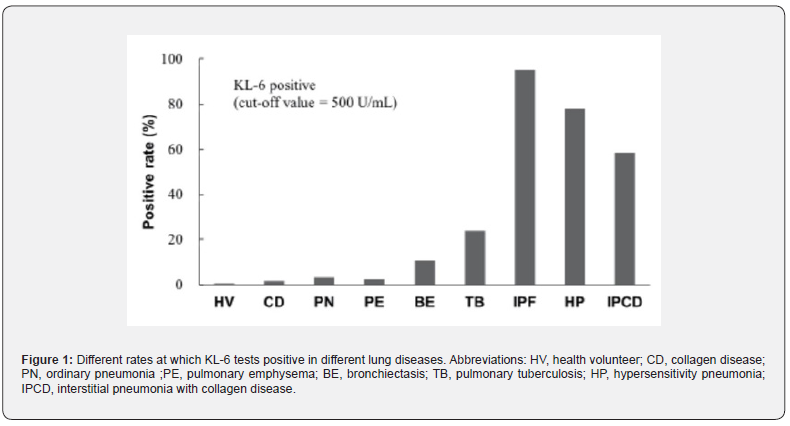
Therapeutic effectiveness is also correlated with serum levels of KL-6. In one trial, high-dose corticosteroid pulse therapy was used to treat 14 Japanese patients with quickly progressing IPF. The patients were monitored for a minimum of three weeks during the treatment. Patients exhibited superior response and longer-term survival when their blood levels of KL-6 dramatically dropped. Numerous investigations revealed a correlation between the degree and activity of IPF and serum KL-6 levels. The prognosis of an IPF patient is also correlated with serum KL-6 levels. There is evidence linking higher blood KL-6 levels, particularly those above 1,000 U/mL at first measurement, to a higher death rate. When KL-6 levels are first measured and they are 1,000 U/mL or above, individuals with IPF progress much more quickly than those whose levels are less than 1,000 U/mL [7].
Surfactant Proteins A and D
In the context of Idiopathic Pulmonary Fibrosis (IPF), Surfactant Proteins A (SP-A) and D (SP-D) emerge as pivotal players in the intricate landscape of pulmonary homeostasis. These hydrophilic proteins, synthesized and secreted by alveolar type II epithelial cells, contribute significantly to the innate immune defense mechanisms within the alveoli. SP-A and SP-D, belonging to the collection family, exert antimicrobial properties by facilitating the opsonization and phagocytosis of pathogens, thereby enhancing host defense against lung infections. Beyond their immunomodulatory roles, emerging research suggests a potential involvement of SP-A and SP-D in modulating the fibrotic process characteristic of IPF. These surfactant proteins regulate inflammatory responses and profibrotic pathways, influencing the delicate balance between tissue repair and aberrant fibrogenesis in the pulmonary microenvironment [8,9].
In the quest for understanding the nuanced interplay of SP-A and SP-D in IPF pathogenesis, ongoing research investigates their potential utility as biomarkers for disease progression and severity. Studies propose that alterations in these surfactant proteins’ expression levels or functional activity may correlate with the extent of fibrotic changes in lung tissue. Furthermore, insights into the genetic variations affecting SP-A and SP-D genes have sparked interest in exploring their association with susceptibility to IPF development and progression. The intricate involvement of SP-A and SP-D in both immune regulation and fibrotic processes underscores their multifaceted role in the complex pathophysiology of IPF, offering avenues for targeted therapeutic interventions and diagnostic advancements [10-12].
Genetic Biomarkers
Genetic Polymorphisms
Lately, studies have made significant strides in understanding the genetic underpinnings of susceptibility to Idiopathic Pulmonary Fibrosis (IPF) and its progression. Notably, some literature delved into studies and found the role of genetic variations in the DPP9 gene in influencing both susceptibility and the rate of disease progression in IPF. The authors identified specific single nucleotide polymorphisms (SNPs) in DPP9, which are connected to an increased risk of developing IPF [13]. The study also found a correlation with a faster decline in lung function among affected individuals [13]. This highlights the dual impact of certain genetic polymorphisms on the progress and initiation of IPF. A study by Fingerlin TE, et al. [14] also boosted our perspective of genetic factors associated with IPF susceptibility. The research employed advanced genomic techniques to identify novel risk loci and revealed previously unrecognized genetic contributors to the disease. Besides, it shows that some of these loci were implicated in pathways related to immune response and lung epithelial cell function [14]. Therefore, the findings contribute to our understanding of susceptibility and open avenues for targeted therapeutic interventions based on the specific genetic factors involved.
Concerning disease progression, a study by Ley B, et al. [15] explored the impact of genetic variants on the rate of decline in lung function among individuals with established IPF. The research focused on comprehensive genetic profiling and identified specific polymorphisms associated with a more rapid fibrosis progression. Understanding the genetic factors influencing disease progression is crucial for developing personalized treatment strategies and predicting outcomes in IPF patients [15]. This assertion is echoed by a study by Mai TH, et al. [16] which depicts that the development of accurate IPF treatment is based on considering multiple factors like gender, which is genotypically determined [16]. Thus, these recent studies underscore the evolving landscape of genetic research in IPF, providing valuable information for understanding susceptibility and tailoring interventions to address disease progression. According to Zhang D, et al. [17] the future of idiopathic pulmonary fibrosis depends on the ability to establish marked genetic variations that help shape interventions and predict disease outcomes [17].
Imaging Biomarkers
High-Resolution Computed Tomography (HRCT)
Idiopathic pulmonary fibrosis, as pathologically defined by the presence of the usual interstitial pneumonia (UIP) pattern, is characterized by distinct radiographic changes [18-20]. The high-resolution computed tomography (HRCT) manifestation of the UIP pattern entails bibasal and peripheral, subpleural reticular opacities, frequently accompanied by traction bronchiectasis-a fibrosis indicator. Linear and reticular opacities result from collapsed alveolar membranes due to scarring. Honeycombing is pivotal for definitively diagnosing idiopathic pulmonary fibrosis [21]. On HRCT, honeycombing is typically described as subpleural, clustered, multilayered, stacked, or multi-tiered cystic air spaces with well-defined walls. These cystic spaces generally range from 3 to 10mm in diameter, occasionally reaching up to 2.5cm, and may signify collapsed secondary pulmonary lobules around respiratory bronchioles. In the most recent consensus classification, HRCT findings are categorized into four types; one is a UIP pattern characterized by peripheral, subpleural, basal-predominant reticular opacities, traction bronchiectasis, and honeycombing, with a typical UIP pattern correlating with UIP pathology in 90 percent of cases; second, a probable UIP pattern featuring the characteristics mentioned above except for honeycombing, corresponding with UIP pathology in 80 percent of cases; third indeterminate for UIP, where the basal, peripheral predominance of opacities is absent, and ground-glass or peri-broncho-vascular opacities are present; and fourth an alternative diagnosis [22].
Diffusing Capacity of Carbon Monoxide (DLCO)
A decline in diffusing capacity (DLCO) is a common observation in idiopathic pulmonary fibrosis (IPF). This decrease results from a ventilation-perfusion mismatch at the alveoli due to thickening from fibrosis at the alveolar-capillary interface [23]. Subsequently, hypoxic vasoconstriction occurs, leading to pulmonary hypertension. Venous occlusive disease follows, causing a reduction in pulmonary capillary blood volume, contributing to a low DLCO in IPF patients [24,25]. Additionally, a decrease in DLCO may occur in IPF patients due to thromboembolic disease [26]. Enhancing DLCO in IPF patients poses a challenge. At the very least, it is crucial to maintain the state of interstitial changes and prevent acute exacerbations.
While drugs approved for pulmonary arterial hypertension (PAH) improve pulmonary capillary blood volume, a persistently low DLCO resulting from alveolar-capillary membrane fibrosis in IPF-PH patients suggests limited improvement in oxygenation [23]. Studies indicate a lower DLCO predicts reduced survival in IPF patients [27]. However, despite large declines (15% or greater) indicating significant disease progression, DLCO’s limited reproducibility and specificity restrict its utility in assessing disease progression over time [28]. The recommendation is to monitor pulmonary function tests (PFTs), including spirometry and DLCO, every three to six months in IPF patients to track disease progression. Further investigations should be prompted to rule out pulmonary embolism, such as high-resolution CT (HRCT) or CT angiography [29].
Molecular Biomarkers
MicroRNAs (miRNAs)
IPF is characterized by a significant involvement of molecular biomarkers, particularly microRNAs (miRNAs). These miRNAs have emerged as potential biomarkers with diagnostic, prognostic, and molecular classification implications in respiratory diseases, including IPF [30,31]. Previous studies have indicated that elevated levels of KL-6 in IPF are predictors of Acute Exacerbations (AE) [30]. Notably, miRNAs, as differentially expressed molecules in respiratory diseases, offer diagnostic value and contribute to prognostic assessments, further enhancing the molecular classification of IPF [31]. A study comparing Bronchoalveolar Lavage (BAL) exosomal miRNAs in IPF has proposed unique miRNA signatures that could serve as airway biomarkers [32].
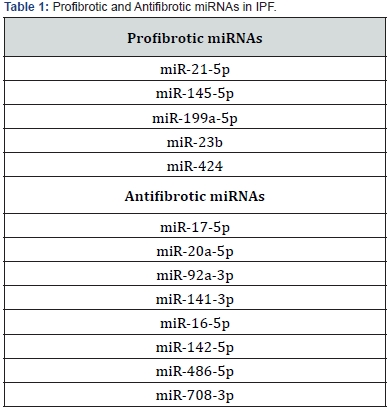
Research by Li H, et al. [31] reported the specific role of miR-26a in IPF. They found that the reduced expression of miR-26a in lung tissues of both mice and IPF patients correlates with TGF-β1 pathway activation and increased expression of the miR-26a target protein CTGF. Inhibition of miR-26a promotes collagen deposition in the lungs of mice, while over-expression inhibits experimental pulmonary fibrosis. Further studies confirm that miR-26a mitigates lung fibrosis by regulating CTGF expression and inhibiting fibroblast differentiation and proliferation [31]. Guiot’s work on miRNA and protein-coding gene expression in IPF identifies 34 overlapping miRNAs, categorized into 7 upregulated (5 profibrotic), 9 downregulated (8 antifibrotic), and 18 with opposite regulation (Table 1). These findings suggest that dysregulation of fibrotic-related miRNAs may contribute to lung fibrotic lesions, particularly in post-COVID-19 patients [33].
Cytokines and growth factors
Growth factors and cytokines are an integral part of the fibrotic microenvironment, which leads to differences in the phenotype of immune cells in the alveoli between patients with pulmonary fibrosis and healthy individuals. Growth factors can participate in the development and progression of IPF in TGF-β-dependent or TGF-β-independent ways. These growth factors comprise platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), connective tissue growth factor (CTGF), and insulin-like growth factor (IGF). Due to the successful marketing of Nintedanib (an antagonist of PDGFR/VEGFR/FGFR), many studies have focused on growth factors and their corresponding receptors.
Th1/Th2 imbalance and M1-M2 polarization are hallmarks of pulmonary fibrosis. Th2 polarization is characterized by increased secretion of IL-4 and IL-13 and decreased secretion of IFN-γ. M2 polarization can be induced by the microenvironment shaped by Th2 polarization and promotes pulmonary fibrosis through the production of TGF-β, CCL18, chitinase 3-like 1 (CHI3L1), MMPs, and activation of the Wnt/β-catenin pathway. Th17 cells can promote fibroblast proliferation and ECM secretion by secreting IL-17. In addition to affecting fibrosis by modulating the Th1/Th2 balance, many studies have shown that interleukins can also directly affect fibroblasts and epithelial cells [34].
Cellular Biomarkers
Circulating Cells
Circulating cells have become a focal point in elucidating the IPF pathogenesis and predicting its progression. These cellular biomarkers, often identified through peripheral blood analyses, offer valuable insights into the systemic manifestations of IPF. Circulating cells, including lymphocytes, monocytes, and neutrophils, undergo phenotypic and functional alterations in response to the fibrotic microenvironment [35]. Recent studies suggest that variations in these immune cells’ proportions and activation states may indicate ongoing inflammation and fibrotic activity within the lungs of IPF patients. For instance, an increased frequency of circulating fibrocytes, a subset of peripheral blood monocytes with fibroblast-like properties, has been implicated in the pathogenesis of IPF, reflecting the intricate interplay between immune responses and fibrosis [35,36]. Furthermore, the identification and characterization of specific subsets of T lymphocytes, such as regulatory T cells (Tregs) and cytotoxic T cells, in the peripheral blood of individuals with IPF contribute to our understanding of the immune dysregulation associated with the disease. The dynamic changes in the circulating immune cell profile underscore the potential utility of these cellular biomarkers as non-invasive tools for disease monitoring and prognosis [37-39].
Conclusion
The comprehensive analysis of predictive biomarkers in Idiopathic Pulmonary Fibrosis (IPF) illuminates a multifaceted landscape where various biomarkers from blood, genetics, imaging, molecular, and cellular domains converge to enhance our understanding of this enigmatic disease. Pursuing accurate predictive biomarkers is imperative given the challenges posed by the insidious nature of IPF, demanding early detection and precise patient stratification for effective intervention. Blood biomarkers like Krebs von den Lungen-6 (KL-6) and Surfactant Proteins A and D (SP-A and SP-D) offer diagnostic precision and prognostic insights, providing a glimpse into the dynamic interplay between immune responses and fibrotic activity. Genetic biomarkers, particularly genetic polymorphisms in genes like DPP9, shed light on the genetic underpinnings of susceptibility and disease progression, paving the way for personalized therapeutic interventions. Imaging biomarkers, exemplified by High-Resolution Computed Tomography (HRCT), unravel the distinct radiographic changes characteristic of IPF, enabling definitive diagnosis.
Molecular biomarkers, such as microRNAs and cytokines, usher in a new era of molecular classification and prognostic assessments, promising tailored interventions based on molecular profiles. Cellular biomarkers, represented by circulating cells, provide non-invasive tools for disease monitoring and prognosis, showcasing the intricate interplay between immune responses and fibrosis. This comprehensive synthesis underscores the collaborative efforts across diverse research avenues to decipher the complexities of IPF. As we navigate this evolving landscape, predictive biomarkers guide the way toward earlier detection, precise stratification, and personalized therapeutic strategies. Integrating these biomarkers into clinical practice promises to transform the management and outcomes of individuals grappling with the challenges of IPF, marking a significant stride in the quest for improved respiratory health.
To Know more about International Journal of Pulmonary & Respiratory Sciences
To Know more about our Juniper Publishers




No comments:
Post a Comment