Cell Science & Molecular Biology - Juniper Publishers
Abstract
The concept of polyherbal formulation (PHF) was initiated via Ayurvedic and other traditional medicinal systems. Additionally multiple plants were used to develop the formulation and evaluated pharmacologically for their synergistic action as well as used to treat many diseases. In the present work we focused on the development and nonpharmacological as well as pharmacological evaluation of PHF on experimental animal model. The plant materials Hibiscus rosasinensis, andrographis paniculata, curcuma longa and moringa oleifera (MO) were collected. Furthermore processed for the evaluation of water soluble extractives, percentage yield, Phytochemical screening, angle of repose, loose and tapped bulk density, carr’s index, hausner’s ratio physiochemical parameters i.e. foreign organic matter, total as value, alcohol soluble extractive value, compressibility index, Hauser ratio, acute toxicity study, anti-analgesic activity such as thermal pain model, mechanical pain model, antipyretic activity, carrageenan induced inflammation as well as antioxidant activity respectively. Phytochemical screening Plant materials were identified and approved by the department of pharmacognosy Columbia Institute of Pharmacy, Raipur, Chhattisgarh, India. The outcomes were found that water soluble extractive of Hibiscus rosasinensis, andrographis paniculata, curcuma longa and moringa oleifera (MO) were 31.09±0.44%, 6.14±.25%, 30.09±0.4% and 19.0±.76%. The percentage yield was 46.03%, 23.30%, 63.39% as well as 30.67% respectively. Moreover, Phytochemical screening of plant materials revealed the presence of Alkaloids, glycosides, saponins, flavonoids, carbohydrate’s, steroid’s, tannins, and phenolic compounds. Data of angle of repose, loose and tapped bulk density, carr’s index, hausner’s ratio were seen 27.03, 0.34, 0.61, 11.97 and 1.11. Additionally acute toxicity was performed as per OECD guideline 423, no weight changes as well as no mortality after 24 h and no behavioral changes observed even at the maximum dose of 4000 mg/kg. Anti-analgesic activity was evaluated as per thermal pain model and mechanical pain model experimental animals were categorized into five groups, Group I treated with normal saline, Group II aspirin 150mg/kg (thermal pain model) & diclofenac sodium 150 mg/kg (mechanical pain model), Group III, IV as well as V were received PHF graded dose reaction time increases with increasing in dose. Antipyretic activity was evaluated on brewer’s yeast induced animal model rectum temperature was reduced with increasing the PHF dose, comparative study was done Paracetamol used as standard. Furthermore, carrageenan induced inflammation was revealed that paw edema size reduced with increasing the dose of formulation. Antioxidant evaluation was done using 2,2-diphenyl-1-picrylhydrazyl radical (DPPH.) assay and oligomeric proantho cyanidins (OPC) was used as a standard, PHF shows antioxidant activity on dose dependent manner furthermore results were shown in the graph a) and b) respectively. The present information on the PHF development and evaluation of the Hibiscus rosasinensis, andrographis paniculata, curcuma longa and moringa oleifera (MO) revealed the qualitative, quantitative, and pharmacological parameters provide the huge information to the identity and to determine the quality and purity of the plant material in the future.
Keywords: Hibiscus Rosasinensis; Andrographis Paniculata; Curcuma Longa and Moringa Oleifera (MO); Polyherbal Formulation; Anti-Analgesic; Anti-Pyretic; Anti-Inflammatory And Antioxidant
Abbreviations: PHF: Polyherbal Formulation; MO: Moringa Oleifera; OPC: Oligomeric Proantho Cyanidins; FOM; Foreign Organic Matter
Introduction
All living things experience pain and inflammation which can be brought on by physical, chemical, or biological factors. Inflammation is often characterized by erythema, swelling, heat, discomfort, and dysfunction. Analgesics are painkillers but they can have several adverse effects such as respiratory depression, apnea, nausea, vomiting, physical and mental illness etc. [1,2]. When used long-term or in high dosage, cell injury and the synthesis of inflammatory mediators such as platelet-activating factors (LTB4) and arachidonic acid prostaglandins (PGE2) in migratory cells are the outcomes of local tissue damage. Neu- trophils and macrophages, which migrate and infiltrate organs, release pro-inflammatory cytokines such as IL-1𝛽 and TNF-𝛼. Folk medicine comprises medical aspects of traditional knowledge that developed over generations within the traditional system beliefs of various societies, including indigenous peoples before the era of modern medicine. While Polyherbal formulation (PHF) is the use of multiple herbs in a therapeutic preparation. The concept was found in ayurvedic and other traditional medicinal systems where multiple herbs in a meticulous ratio may be used in the treatment of diseases [3]. Nowadays most diseases possess due to excessive prostaglandins and oxidants produced in the body which modifies physiology of body. The management of prostaglandins and antioxidants through the herbal’s formulation is a rational approach to preventive health care. The importance of disease prevention is recognized by ayurveda through experience accumulated over centuries. Hibiscus rosasinensis, andrographis paniculata, Curcuma longa and moringa oleifera (MO) in the ratio of 1:1:1:1. Antioxidant and immune-stimulating effects of M.O. have already been established in animal models [4].
Materials and Methods
Drugs and Chemicals used
Methanol 95%, normal saline (Merck Ltd India), acetic acid (New arihant chemicals mumbai ,india,), brewer’s yeast and carrageenan (Bayers Pvt Ltd). Tramadol, aspirin, diclofenac sodium, paracetamol (Cipla Pharmaceutical Industry India) and other chemicals were used as well as procured of analytical grade.
Collection, Identification and Authentification
The fresh flowers, leaves, roots, and fruits of Hibiscus rosasinensis, andrographis paniculata, curcuma longa and moringa oleifera (MO) were collected in june, 2021 from Raipur, Chhattisgarh, India. The plants were identified and authenticated by Dr. S. Prakash Rao, Department of Pharmacognosy, Columbia Institute of Pharmacy, Raipur, Chhattisgarh, India, 492001.
Physiochemical Evaluation
All plant parts were dried, crushed and converted into fine powders with the help of mixer and grinder there after quality assessment of plant materials were done as per the standard protocols of Ayurvedic Pharmacopeia of India (API). Different parameters were tested with the methods described in API [5].
Foreign Organic Matter (FOM)
As per API, FOM is described as any material that consists of part of organ or organ part from which the drug is derived. The plant should be free from any unfamiliar particle like dust, insects, faecal matter etc. The percentage of foreign matter should not be more than the limit prescribed in monograph. There should not be any contamination in drug material used for PHF [6].
Procedure
750 gm of plant materials were weighed and spread as a thin layer and was inspected first with naked eyes thereafter by using lens (6x). The entire FOM were Separated, weighed and percentage was deliberated.
Determination Of Total Ash Value
3.0 gm of dried powered mixed sample was weighed in silica dish, and it was incinerated at a temperature not exceeding 450°C until it gets free from carbon. The incinerated material was cooled, weighed and percentage of ash was calculated with reference to air dried drug. The determination of Acid insoluble ash value Ash obtained was boiled with 25 ml of dil. HCL for 5 minutes filtered and insoluble matter was collected in crucible and washed with hot water and ignited till constant weight. The percentage of acid insoluble ash was calculated with respect to air dried drug [7].
Determination of Alcohol Soluble Extractive Value
5gm of powdered drug was macerated with 100 ml of alcohol in cork fitted conical flask. Solution was shaken frequently for 6 hrs and was allowed to stand for 18 hrs. After 18 hr. content was filtered and 25 ml of filtrate was evaporated to dryness in a shallow dish at 105°C to constant weight and percentage of alcohol soluble extractives was calculated with reference to air dried drug.
Determination of Water-Soluble Extractives
5gm of powdered drug was macerated with 100 ml of water in cork fitted conical flask. Solution was shaken frequently for 6 hrs and allowed to stand for 18 hrs. After 18 hr. content was filtered and 25 ml of filtrate was evaporated to dryness in a shallow dish at 105°C to constant weight and percentage of water-soluble extractives was calculated with reference to air dried drug. The data generated in respect of the above findings will be used as in-house standards.
Preparation of Hydro-Alcoholic (HA) Extracts and Percentage Yield Estimation
Dried powder was processed in order to prepare the PHF, about 750gm of Hibiscus rosasinensis (flower, leaves), 750gm of andrographis paniculata (flower, leaves, roots), 750gm of curcuma longa (flower, roots) and MO (flower, leaves, fruits) powders were soaked overnight separately in 1500ml of Petroleum Ether (PE). After 3 days the suspension was filtered, and PE was to be evaporated overnight. Again, the dried powders were separately resuspended in a Stoppered container with the HA solvent. Allowed to stand at room temperature (RT) for a period of 7days. Additionally, extract was concentrated to dryness in a rotary evaporator (Buchi type) under reduced pressure and controlled temperature (37-40℃) to get percentage yield [8].
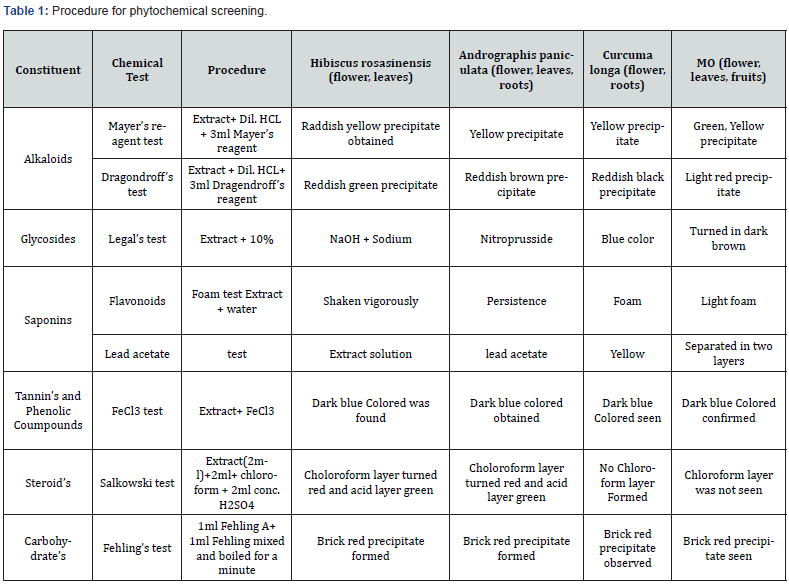
Phytochemical Screening
Crude extract of plants was subjected to different chemical tests to detect the presence of various phytochemical constituents as per procedure adopted in literature. The details are incorporated below in table 1, the entire chemical test was discussed in results table 2,3 [9].
Design and Development Of PHF
Extracts of four plants Hibiscus rosasinensis, andrographis paniculata, curcuma longa and moringa oleifera (MO) formulation have been made by blending the extracts in ration 1:1:1:1. Evaluation of Polyherbal formulations Prepared PHF was evaluated on following parameters [10].
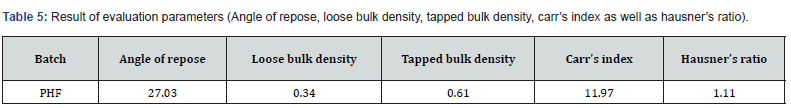
Angle Of Repose
The angle of repose was determined by using the funnel method. The accurately weighed blend was taken in a funnel. The height of the funnel was adjusted in such a way that the tip of the funnel just touches the apex of the heap or head of blend. The drug excipient blend was allowed to flow through the funnel freely onto the surface. The diameter of the powder cone was measured, and angle of repose was calculated using the following equation:
tan θ = h/r
Where, h = height of powder cone formed, r = radius of the powder cone formed
Loose Bulk Density
Apparent bulk density was determined by pouring a weighed quantity of blend into graduated cylinder and measuring the volume and weight.
LBD = Weight of the powder / volume of the packing.
Tapped Bulk Density
It was determined by placing a graduated cylinder, containing a known mass of drug excipient blend. The cylinder was allowed to fall under its own weight on to a hard surface from the height of 10cm at two second intervals. The tapping continued until no further change in volume was noted.
TBD = Weight of the powder/vol of the tapped packing
Compressibility Index
The Compressibility index of the powder was determined by Carr’s compressibility index.
Compressibility index (%) = (TBD-LBD) x 100/TBD
Hausner Ratio
It is the measurement of frictional resistance of drug and ideal range should be 1.2-1.5. It is determined by using the following formula:
Hausner ratio= TBD / LBD
Acute Toxicity Study of PHF As Per OECD Guidelines
Preparation of Formulations
For dosing 100 ml of each formulation was prepared by dissolving 5gm of formulation in 100 ml of distilled water (So, 1ml contain 50 mg of drug).
Experimental Animals
Healthy male albino wister rat were taken weight ranging from 175-250gm. The animals were procured and kept at the animal house of the Columbia Institute of Pharmacy, Raipur, Chhattisgarh, India. Animals were reserved in a neat and clean atmosphere in polypropylene cages, under 12 h light and dark cycles. The room temperature (RT) was maintained at 24 ± 0.40C with controlled humidity. Animals were fed in strict hygienic conditions with a rodent pellet diet and water ad libitum. The experiments on animals were performed and approved as per the guidelines of the Animal Ethics Committee. All the animal experiments were carried out according to the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines, Regd. No. 0310/PO/ReBi/S/20/01/CPCSEA.
Acute Toxicity Study of PHF
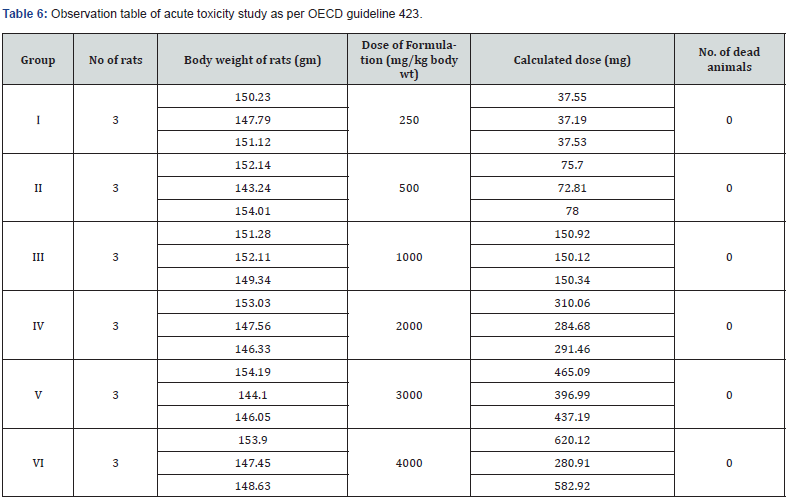
Acute toxicity studies were carried out in adult female albino rats weighing between 130-180 gm by Acute Oral Toxicity method of OECD Guideline 423. They were administered (orally) with varying doses (250, 500, 1000, 2000, 3000 and 4000 mg/kg body weight) for each of six formulations. Animals were divided into 6 groups of three animals each and were acclimatized for 5 days. Prior to dosing animals were kept fasted overnight and next day each formulation was administered orally at a dose level of 250, 500, 1000 and 2000 mg/kg body weight. Rats were observed for clinical signs of toxicity continuously for 2 hours and occasionally for further 4hours for general behavioral and finally for any mortality after 24 hours till 14 days. No mortality was observed during a time period of 14 days [11].
Analgesic Activity
Thermal Pain Model (Tail Flick)
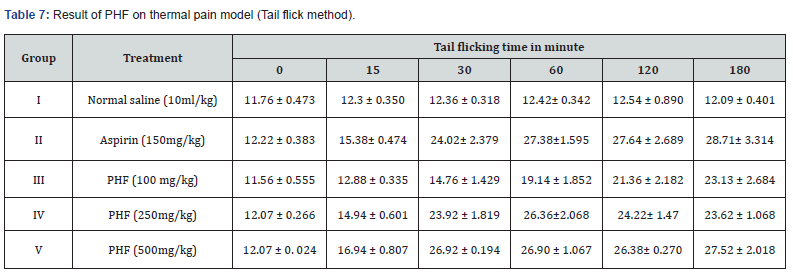
Rats were categorized in Group I (Control: Normal saline), Group II treated with Aspirin, 150 mg/kg; Group III, IV & V were given PHF treatment 250 and 500 mg/kg orally one hour before beginning of the experiment. The tip of the tail was immersed in tail flick algesimeter (50.0 ± 1.0 °C) and observed for response. Data were noted at 0, 15, 30, 60, 120, and 180 min in tabular format.
Mechanical Pain Model (Eddie’s Hot Plate)
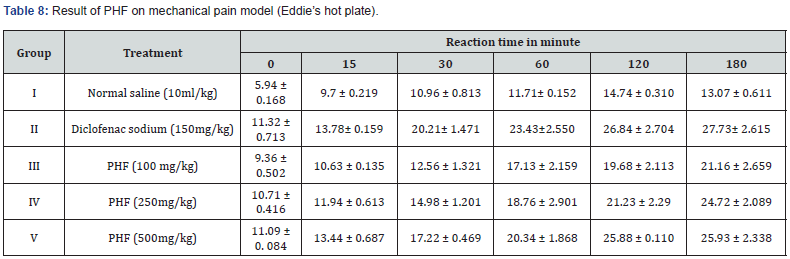
Animals were grouped consequently Group I (Control: Normal saline), Group II Diclofenac sodium (150 mg/kg); Group III, IV & V treated with PHF 250 and 500 mg/kg. We placed the rats’ right paws on the eddies hot plate and spontaneous withdrawal reflex was recorded at 0, 15, 30, 60, 120, and 180 min respectively.
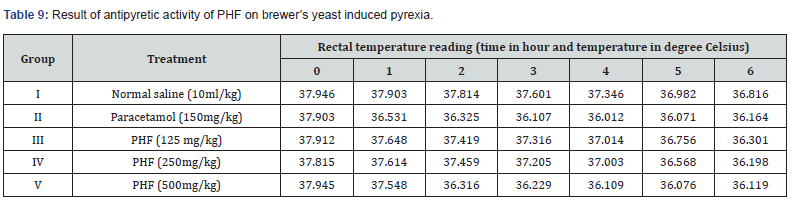
Antipyretic Activity
In this present work we used Brewer’s pyrexia for the elevation of body temperature in animals. They received 20% w/v of brewer’s yeast suspension 10 ml/kg. Furthermore, animals were randomized into five groups, Group I was considered as controlled group, Group II was treated with 150mg/kg paracetamol, Group III, IV & V were given 125, 250 and 500 mg/kg of PHF. The temperature of rectum was noted at the time interval of 0, 1, 2, 3, 4, 5 and 6 h respectively.
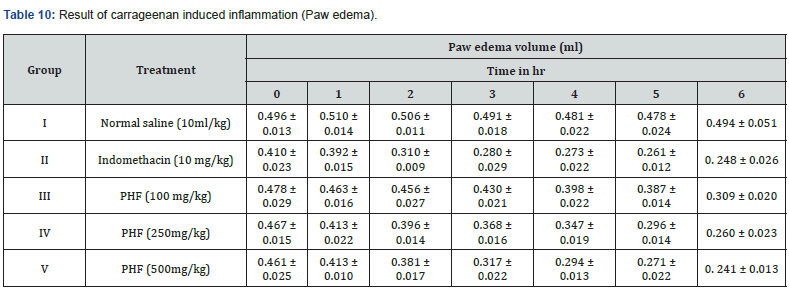
Carrageenan Induced Inflammation (Paw Edema)
Animals were grouped accordingly Group I Control (Inflammation control), Group II was treated with Indomethacin 10 mg/kg and Group III, IV & V were given orally 100, 250 and 500 mg/kg of PHF 30 minute earlier than carrageenan injection. Moreover, each animal’s left paw was injected 2.0% w/v carrageenan after 0, 30, 1, 2, 3 and 4h every rat’s paw volume was measured carefully using water paleothermometer. Size of edema were noted down by means of A and B where A is before the inducing agent treatment as well as B is after induction [12].
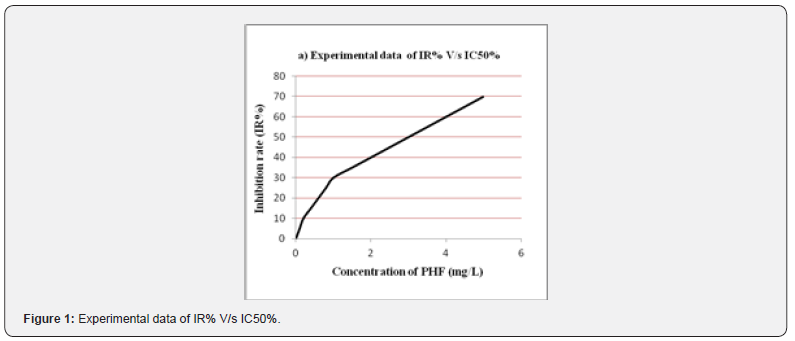
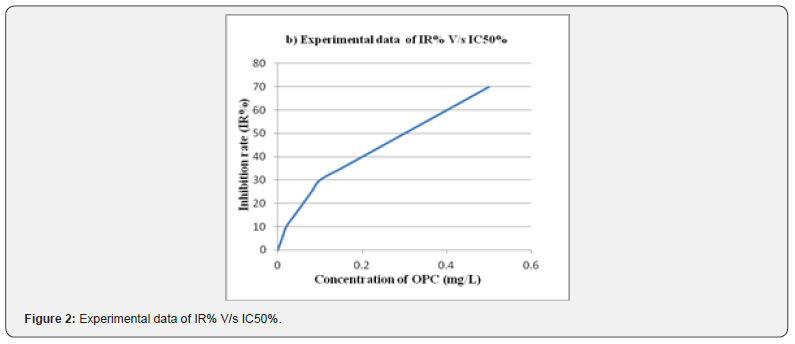
Antioxidant Activity
The free radicals scavenging property of PHF was determined by 2,2-diphenyl-1-picrylhydrazyl radical (DPPH.) assay and oligomeric proantho cyanidins (OPC) was used as a standard for the comparative study of PHF extract. Furthermore, a 50% inhibition rate in the corresponding concentration is known as IC50. Hence the significance of IC50 was calculated by comparing the values of sample concentration and inhibition rate of PHF [13].
Result and Discussion
Physiochemical Evaluation Of PHF
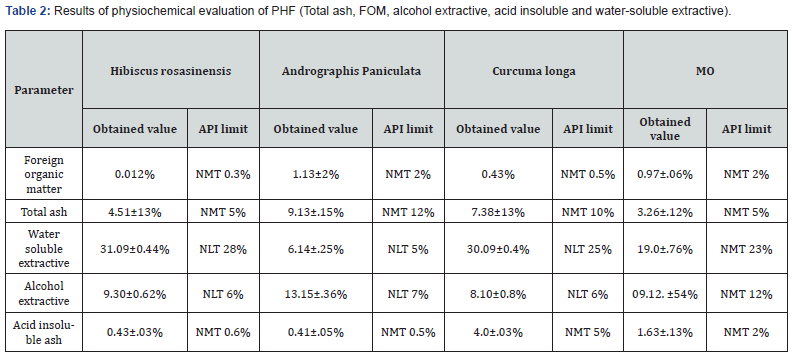
The data of all determinations were summarized in Table 1, parameters incorporated such as foreign organic matter, total ash, acid insoluble ash, alcohol as well as water soluble extractive of PHF; preparation was found under ayurvedic pharmacopoeia confines. (Table 2)
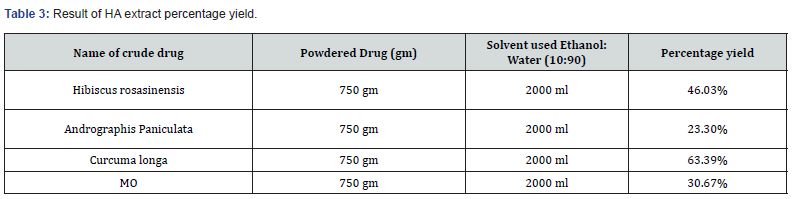
Percentage Yield of HA Extract
HA extract of powdered crude drug were obtained after soaking for the three days. Furthermore, processed through the butchi rotavapour, percentage yield statistics information was given in Table 3.
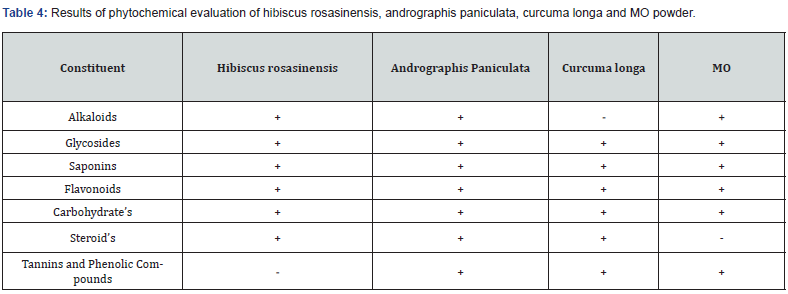
Phytochemical Evaluation Of PHF
Extract of crude drugs was processed for some special identification tests to distinguish the presence of different chemical components. Additionally, evaluation parameters i.e., Alkaloids, glycosides, saponins, tannins, phenolic Coumpounds, steroid’s as well as Carbohydrate’s result were described in Table 4.
Design, Development, and Evaluation Parameters Of PHF
PHF was prepared as per standard technique as per previously reported in literature, which targeted each site in the body. Furthermore, formulation is helpful in minimizing analgesic, pyretic, inflammation as well as having strong ability to scavenge free radicals. Additionally evaluated on the parameters i.e., Angle of repose, loose bulk density, tapped bulk density, carr’s index as well as hausner’s ratio respectively figures were given in Table 5.
Acute Toxicity Study of PHF
Phytochemical screening of PHF established that it be full of Alkaloids, glycosides, saponins, flavonoids, carbohydrates, steroid’s, tannin’s along with phenolic compounds as per API. Furthermore, acute toxicity study of PHF was estimated as per OECD guideline 423, formulation lead to no weight changes as well as no mortality after 24 h and no behavioral changes observed even at the maximum dose of 4000 mg/kg statistical analysis given in observation Table 6.
Evaluation of Anti-Analgesic Activity
Thermal Pain Model (Tail Flick)
Throughout the observation period normal saline, aspirin 150 mg/kg and PHF graded dose 100, 250 and 500mg/kg were administered. Values were compared as well as revealed that tail flicking time is directly proportional to dose, given in Table 7.
Mechanical Pain Model (Eddie’s Hot Plate)
During the 180h of observation period experimental animals were administered normal saline 10ml/kg, diclofenac sodium 150mg/kg and PHF 100mg/kg, 250mg/kg as well as 500mg/g body weight. Consequences observed that reaction time increases in dose dependent manner, given in Table 8.
Evaluation of Antipyretic Activity
Pyrexia was induced by brewer’s yeast, reduction of rectal temperature by different doses of paracetamol and PHF were noted on Table 9. Furthermore, evidence was seen that PHF reduces body temperature in a dose dependent manner.
Carrageenan Induced Inflammation (Paw Edema)
The experimental animals were induced using carrageenan as well as treated with normal saline 10ml/kg, indomethacin 10 mg/kg and PHF 100, 250 and 500 mg/kg body weight respectively. Hence, results were elevated that size of induced paw edema gradually decreases in the dose dependent manner; values were given in Table 10.
Evaluation Of Free Radicals Scavenging Property
The PHF of Hibiscus rosasinensis, andrographis paniculata, curcuma longa and MO confirmed that the scavenging of DPPH free radicals and a maximum scavenging activity was depends on the concentration. Still data were recorded at a concentration of 1, 2, 3, 4, 5 and 6 mg/L PHF, respectively. OPC, with the strong ability to scavenge free radical, was used as the standard to estimate the antioxidant activity of PHF. As per (Figure 1,2) the assessment of IR greater than before along with the concentration added. Furthermore, the IC50 value of PHF and OPC were established that 3.0mg/L as well as 0.3mg/L respectively. Therefore, the antioxidant activity of extracts from 1 mg PHF was approximately equal to 0.1 mg OPC.





No comments:
Post a Comment