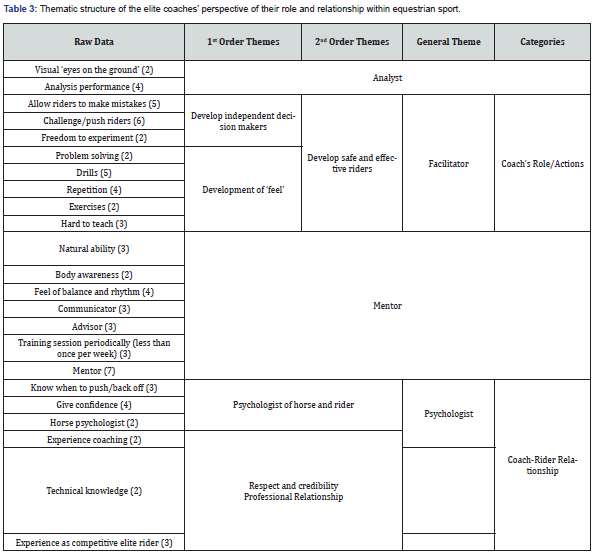
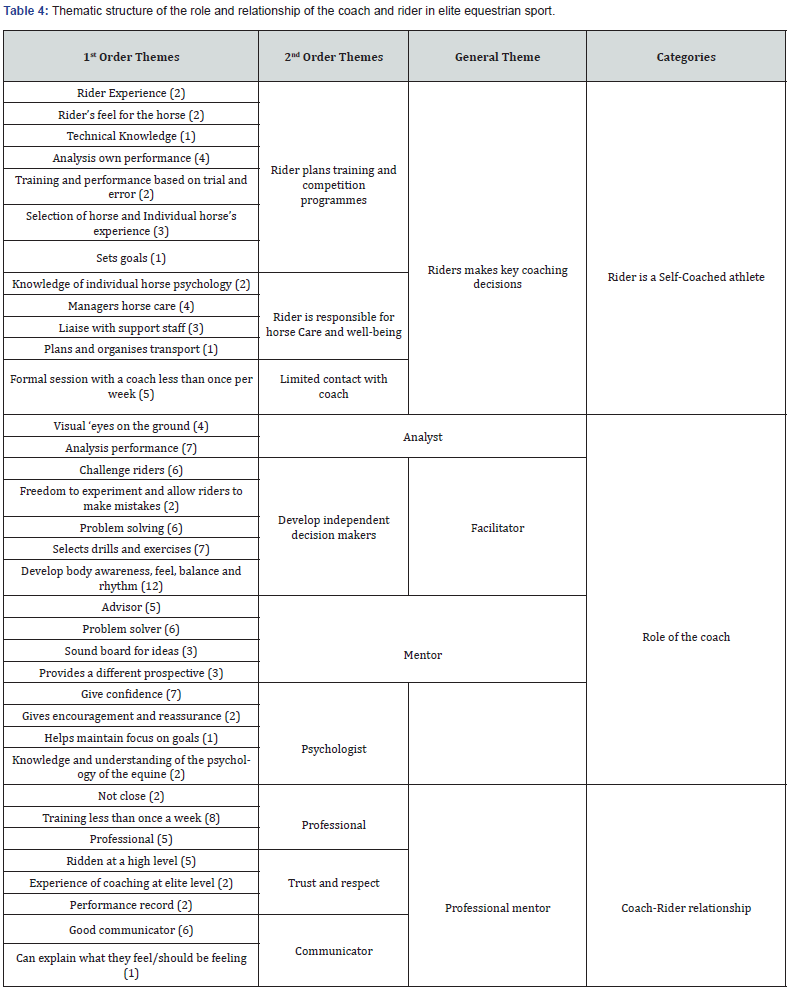
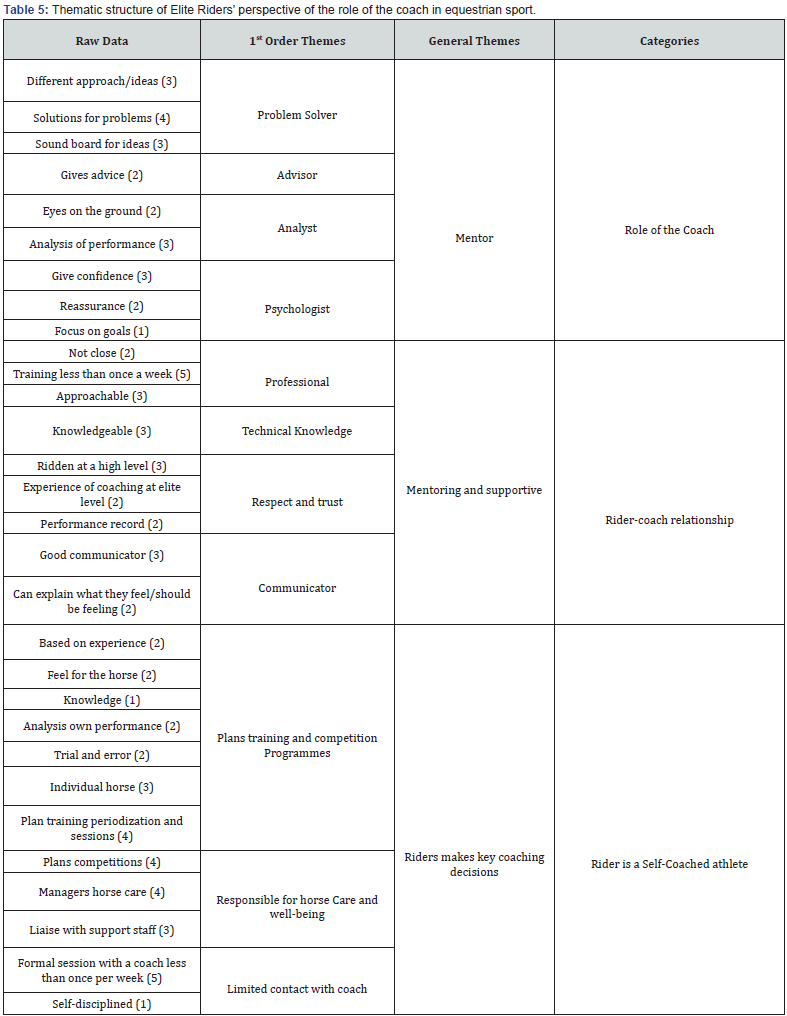
Pharmacology & Clinical Research - Juniper Publishers
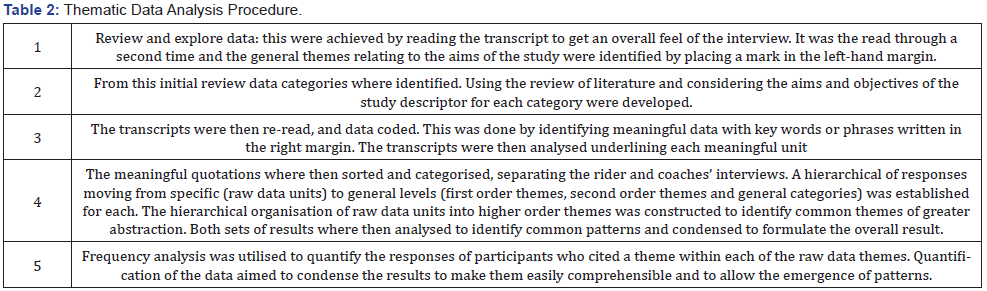
Abstract
Purpose: Phenylephrine hydrochloride (HCl) is a commonly used adjuvant in anesthesia for the treatment of hypotension, as well as, shock and shock-like states. Phenylephrine HCl is manufactured in a highly concentrated form, and therefore must be diluted before use in patients. Phenylephrine HCl is commonly diluted into 100 mL 0.9% normal saline bags, ensuring the dilute form is readily available if an urgent or emergent situation occurs. A 100 mL bag of dilute phenylephrine is likely excessive for one patient, and thus may be used on multiple patients provided the provider ensures proper aseptic technique is utilized. The purpose of this study was to examine the stability of phenylephrine in polyvinyl chloride (PVC) bags. Methods. Phenylephrine HCl 10 mg/mL solution was diluted with 0.9% sodium chloride for injection to a final concentration of 100 μg/mL and stored at room temperature (23°C - 25°C) exposed to fluorescent light, room temperature (23°C - 25°C) exposed to dark, 4°C exposed to dark, and 52°C exposed to dark. Stability of phenylephrine HCl was evaluated by high-performance liquid chromatography (HPLC) on days 0, 15, 30, and 138. Results. Phenylephrine HCl diluted to 100 μg/mL with 0.9% sodium chloride injection was physically stable throughout the study, except when it was exposed to fluorescent light. Phenylephrine HCl retained ≥ 90% of the original concentration when it remained in the dark at 4°C, room temperature, and 52°C. However, Phenylephrine HCl degraded by >35% when it was exposed to normal fluorescent light at room temperature. When exposed to fluorescent light, phenylephrine HCl discolored over time and developed a slightly turbid appearance. Conclusions. Phenylephrine HCl diluted to a concentration of 100 μg/mL in 0.9% sodium chloride was stable for at least 138 days with ≤10% degradation when stored in PVC bags in the dark at 4°C, room temperature (23°C - 25°C), and 52°C. Phenylephrine HCl diluted to a concentration of 100 μg/mL in 0.9% sodium chloride was not stable when exposed to fluorescent lighting at room temperature.
Keywords:Phenylephrine Hydrochloride; Fluorescent Light; Vasoconstriction; Fluorescent Lighting; Anesthesiologists; Paroxysmal Supraventricular Tachycardia
Abbreviations:FDA: Food and Drug Administration; HCL: Hydrochloride; PVC: Polyvinyl Chloride; HPLC: High-Performance Liquid Chromatography; IV: Intravenous; PVC: Polyvinyl Chloride
Introduction
Chemical stability of pharmaceutical molecules is an important physicochemical property as it affects the safety and efficacy of drug products. The Food and Drug Administration (FDA) states that there is a regulatory requirement of stability testing data to understand how the quality of a drug substance and drug product changes with time under the influence of various environmental factors [1-4]. Information regarding the stability of a drug helps in determining the required formulation and storage conditions to maintain efficacy and safety measures as well as the desired shelf life. Drug products are known to be affected by a variety of environmental factors such as temperature, humidity and light as well as product related factors such as chemical and physical properties of active substance and of pharmaceutical excipient, the dosage form and its composition, the manufacturing process, the nature of the container closure system and properties of packaging material [5-6]. Thus, the stability study is essential not only for regulatory approval but also ensures safety of the patient. Phenylephrine hydrochloride (HCl) is a synthetic sympathomimetic agent similar to epinephrine and ephedrine [7]. Its potent alpha-1-adrenergic-agonist properties lead to vasoconstriction and increased perfusion pressure. For this reason phenylephrine is commonly used by anesthesiologists to increase blood pressure during surgical procedures [8-11]. In addition to the maintenance of an adequate level of blood pressure, it is used for the treatment of vascular failure in shock, shock-like states, and drug-induced hypotension, or hypersensitivity. It is also employed to overcome paroxysmal supraventricular tachycardia, to prolong spinal anesthesia, and as a vasoconstrictor in regional analgesia [12].
Phenylephrine can be administered as an intravenous (IV) bolus dose or a continuous IV infusion. To prepare phenylephrine for administration, concentrated stock phenylephrine vials must be further diluted. Pharmacies commonly prepare phenylephrine hydrochloride 100 μg/mL syringes for use by anesthesiologists. Parenteral products in solution form need to be tested to ensure its purity, safety and accuracy when used in a clinical setting as storage conditions may impact pH, temperature, light and oxidation which are the critical factors for drug product degradation [13,14]. Previous stability studies have shown phenylephrine to be stable for up to 30 days at room temperature when diluted to 100 μg/mL in sodium chloride 0.9% in polyvinyl chloride (PVC) bags, and up to 60 days when diluted to 200 and 400 μg/mL [15, 16]. However, no studies have evaluated the stability of phenylephrine stored in PVC bags for as long as 138 days. The purpose of this study was to determine the physical and chemical stability of phenylephrine diluted to 100 μg/mL in 0.9% sodium chloride PVC bags when stored using four different conditions. The first condition was at room temperature (23°C - 25°C) in a dark drawer (RT dark), while exposed to minimal amounts of fluorescent lighting. The second condition was room temperature (23°C - 25°C), exposed to constant ambient light, including fluorescent lighting (RT light). The third and fourth conditions were stored in the dark at 4°C and 52°C respectively, while exposed to minimal amounts of fluorescent lighting. Stability of the diluted phenylephrine solutions was evaluated over a 138-day period.
Methods
Sample Preparation
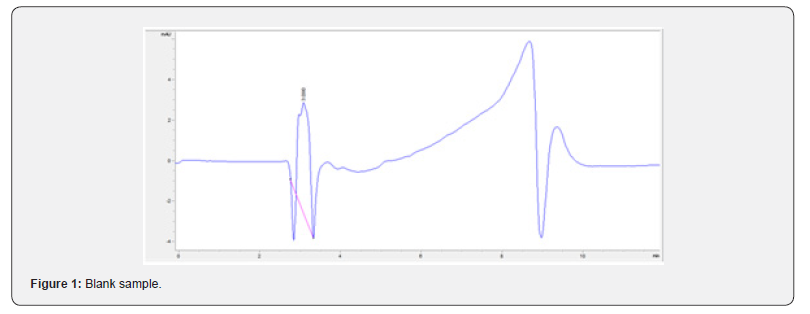
Dilution of phenylephrine hydrochloride to 100 μg/mL was performed under aseptic technique by adding 10 mg of phenylephrine to 100 mL 0.9% sodium chloride PVC bags. The bags were then stored at their respective storing conditions: 52°C water bath, room temperature (23°C - 25°C) exposed to direct fluorescent light, room temperature (23°C - 25°C) with no light, and refrigerated at 4°C. Four different bags corresponding to different storage conditions were assessed for physical and chemical stability over a total period of 138 days. Stabilities were assessed on a short-term (days 0, 15, 30) and long-term (day 138) basis (Figure 1).
Physical Evaluation
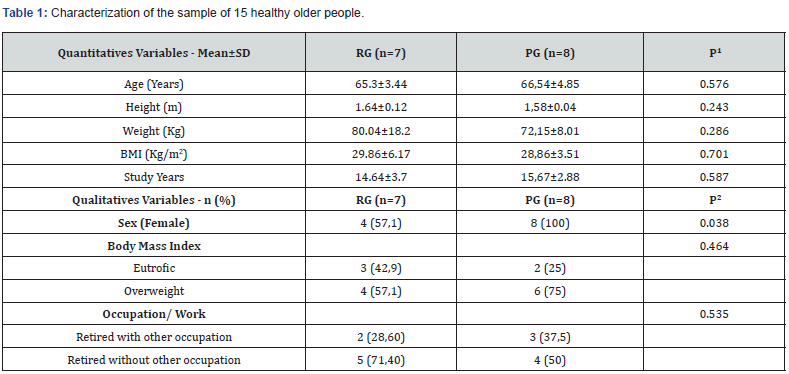
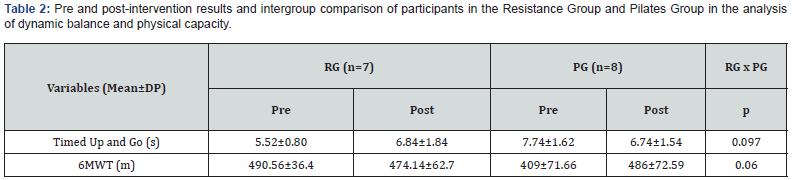
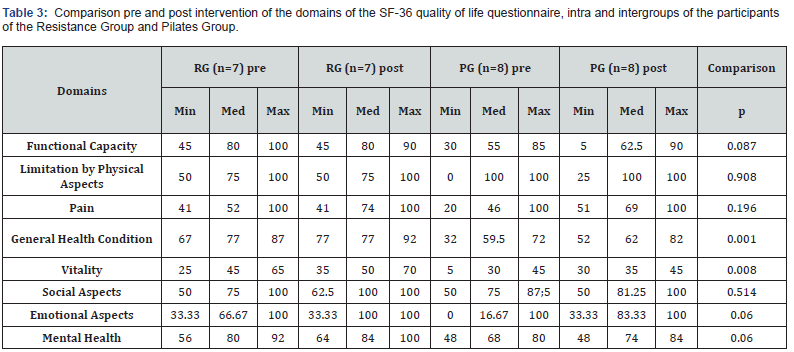
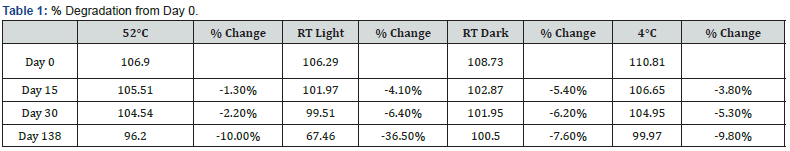
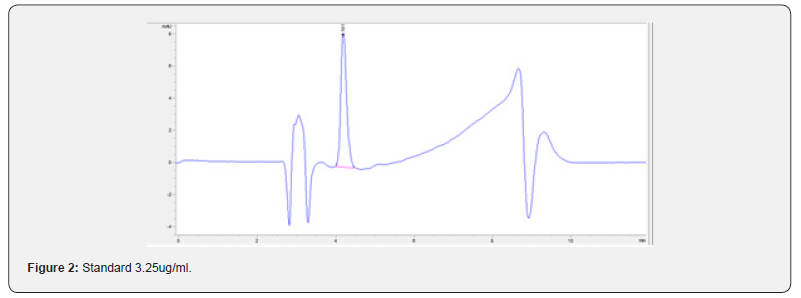
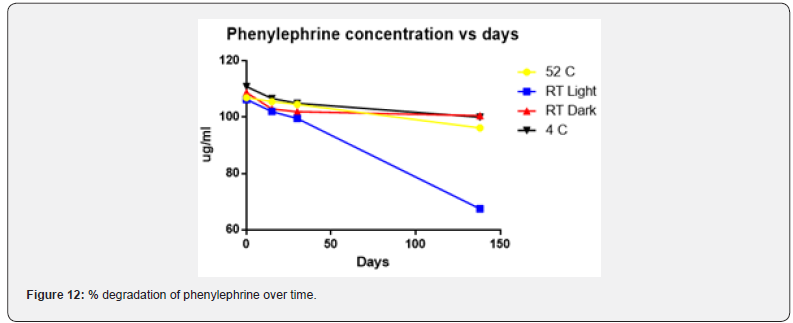
Physical stability of phenylephrine was assessed by visual examination. Solutions were evaluated against a black and white background for visible particulate matter, cloudiness, or color change. The pH of the samples was not assessed, as previous studies have not demonstrated appreciable changes in pH. Phenylephrine concentrations were determined using highperformance liquid chromatography (HPLC) with ultraviolet light. The HPLC system consisted of an autosampler, a delivery pump, a C18 column with a guard column, a variable-wavelength ultraviolet light detector set at 273 nm, a data integrator, and a solvent waste container. A gradient mobile phase system consisting of 0.02M ammonium acetate with 0.1% formic acid (A) and methanol with 0.1% formic acid (B) was used. The separation was achieved with a linear gradient program as follows: 20% v/v B at zero time; from 0 to 5 min, ramp up to 67% v/v B; from 5.01 to 20 min, hold 20% v/v B. The flow rate was 1.5 mL/min. The injection volume was 100 μL. The chromatograms were extracted at 273 nm. All determinations were performed at 25°C. The HPLC software, Chem Station (Version C.01.09; Agilent, 2018) was used to perform the regression analysis, generate the best-fit equations, and generate the coefficient of correlation values. Chromatogram peak heights and areas were used to determine phenylephrine concentrations. All samples for each storage condition were assayed in duplicate. Calibration of the HPLC system was performed by construction of a standard curve using nine known concentrations of phenylephrine hydrochloride (range, 3.125– 200 μg/mL) (Figures 2-10). Coefficients of determination (R2) for the standard curve were 0.995–0.999 for the entire study. Retention time for phenylephrine was on average around 3-4 minutes. Phenylephrine diluted to 100 μg/mL in 0.9% sodium chloride for injection was physically stable in all conditions except for room temperature (23°C - 25°C) exposed to fluorescent light. When exposed to light at room temperature, the solutions changed from clear to a light red hue color with a slightly turbid appearance around day 108. Approximately, on average 7.6% to 10.0% phenylephrine degradation was observed over the 138- day study in all conditions except the room temperature exposed to fluorescent light which experienced a 36.5% degradation (Table 1).
Study Limitations
The study was originally designed to determine long-term stability at Day 120. However, we encountered a technical problem whereby the HPLC column experienced catastrophic failure after day 30. Several attempts to repair the column were performed including extended wash cycles and regeneration cycles. These attempts were unsuccessful and had to resort to ordering a duplicate HPLC column with the same characteristics as the original one. The duplicate HPLC column had to undergo several quality control tests to ensure reliability prior to using it for this experiment. Consequently, the duplicate HPLC column was verified to be operational on day 137 and therefore the longterm stability testing date was performed on the following day (day 138) (Figure 11 & 12).
Discussion
Previous studies have evaluated the stability of phenylephrine in 0.9% sodium chloride solution [14-16]. A study by Gupta [14] found that phenylephrine was stable in 0.9% sodium chloride solution for 14 days. This study found less than 1% degradation of phenylephrine stored in PVC bags using HPLC analysis after the 14-day period [14]. While the Gupta [14] study showed almost no degradation (<1%) of phenylephrine exposed to light, the current study revealed a 4.07% degradation of phenylephrine exposed to light at 15 days. The Kiser [15] study found that phenylephrine remained stable for 30 days while exposed to normal fluorescent lighting. All samples involved in the Kiser study experienced less than 2% degradation over the 30-day study period [15]. Despite a slightly higher degradation rate of 2.2% to 6.4% at the 30-day point, the current study also showed stability of phenylephrine in solution at 30-days. A 60-day study by Jensen [16] demonstrated the stability of phenylephrine to continue over the course of the study. The Jensen study showed a 1% to 3% degradation at 30 days, and about 4% to 6% degradation at 60 days [16]. The current study suggests that phenylephrine remains stable in solution for at least 138 days when stored in the dark. Similar to previous studies it also suggests that phenylephrine remains stable for at least 60 days when a room temperature sample is exposed to normal fluorescent lighting. This study did not look at the sterility of phenylephrine in solution. Phenylephrine manufacturers do not include preservatives within the phenylephrine vials, and the sterility of prepared solutions of phenylephrine must be assessed according to the standards in chapter 797 of the United States Pharmacopeia [17]. Further research is needed to assess the sterility of phenylephrine solutions for long-term use. Knowledge of the extended stability and storage conditions of phenylephrine in solution in PVC bags allows individual practitioners and pharmacies to prepare solutions in advance and store them until the drug is clinically needed. This allows immediate access and reduced delivery time to a potentially life-saving drug in the event of a hypotensive emergency. Additionally, the extended stability of phenylephrine in solution could potentially reduce drug costs and wastage over time.

Conclusion
This study suggests that phenylephrine hydrochloride diluted to a concentration of 100 μg/mL in 0.9% sodium chloride remains stable for at least 138 days for samples that are stored in the dark. Similar to previous studies this study also suggests that phenylephrine hydrochloride diluted to a concentration of 100 μg/mL in 0.9% sodium chloride exposed to normal fluorescent lighting is stable for at least 60 days at room temperature.