Reproductive Medicine - Juniper Publishers
Abstract
MKS, Meckel Gruber syndrome is a rare, lethal, genetic multiple congenital anomaly characterized by the following triad: cerebral malformation (primarily occipital encephalocele), polycystic kidneys, and polydactyly, as well as associated abnormalities that may include cleft lip/palate, cardiac and genital abnormalities, central nervous system (CNS) malformations, hepatic fibrosis, and bone dysplasia.
The mode of transmission of MKS is autosomal recessive. Genetic counseling should be offered to couples at risk. MKS is lethal in utero or in the very early neonatal period, with pulmonary hypoplasia and renal failure being the main causes of early death. Our case illustrates the importance of prenatal diagnosis, essentially by ultrasound, in order to ensure adequate management.
Keywords: Meckel syndrome; Renal dysplasia; Encephalocele; Antenatal diagnosis; Polydactyly
Abbreviations: CNS: Central Nervous System; AR: Autosomal Recessive; MKS: Meckel-Gruber Syndrome
Introduction
Meckel-Gruber Syndrome, is a rare, lethal, autosomal recessive congenital polymalformative syndrome, which was first described in 1822 in the German literature [1]. MKS was described by Meckel in 1822, and Gruber in 1934 combining encephalocele, cystic dysplasia of the kidneys, and polydactyly. The variability of the clinical cases reported in the literature demonstrates that the multifaceted nature of this syndrome is an essential characteristic. Ultrasound is, at present, the best means of antenatal diagnosis of this lethal poly malformation [2]. The diagnosis is based mainly on the genetic study especially karyotype, thus allowing to separate the differential diagnosis especially trisomy and 13,18, as it also allows to assign the genetic counseling to the couple. In this article we report a case of Meckel-Gruber syndrome in a young couple with notion of consanguinity, who benefited from an echography within the framework of screenings of malformation making suspect this syndrome before the encephalocele and polycystic kidneys, the pregnancy stopped at 28 weeks of amenorrhea.
Case Presentation
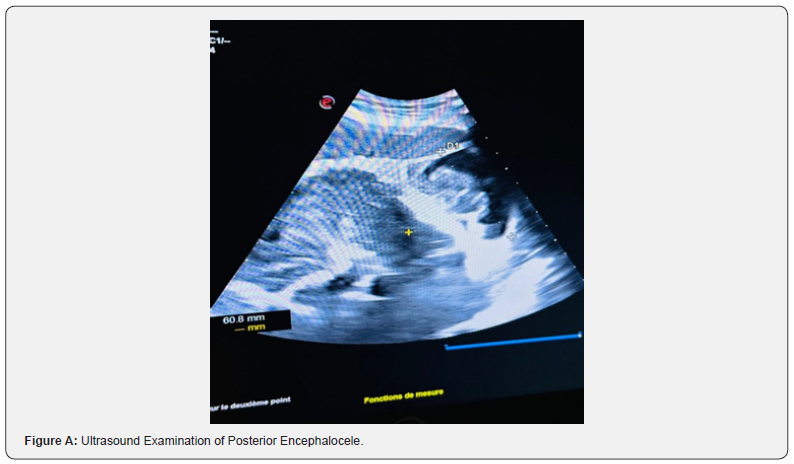
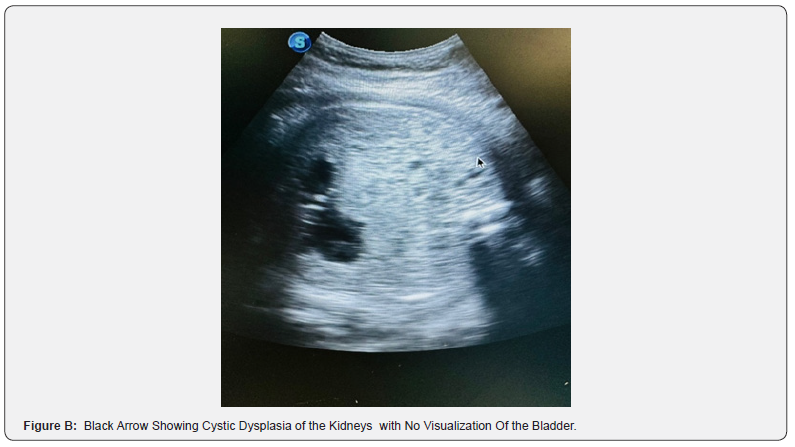
We hereby present the case of a 20-year-old woman, without any particular pathological history, primigravida primiparous, with notion of 1st degree consanguinitis, the patient was followed at our facility and underwent a first-trimester ultrasound examination at 14 weeks of gestation, showing occipital encephalocele (Figure A), hydrocephalus, renal dysplasia and non-visualized bladder (Figure B), raising strong suspicion of MKS syndrome. The prenatal check-up was without abnormalities, and there was no evidence of any drug or Phyto-therapeutic intake. The medical interruption of pregnancy was proposed and refused by the couple.
At 28 weeks of gestation the patient was referred in labor to our structure, the ultrasound on admission showed the same polymalformative complex associated with total amnion, she gave birth to a newborn female, weighing 1700g who died after 6 hours of life due to respiratory distress. The macroscopic inspection revealed: on the cephalic pole: retrognatitis (Figure 1) and posterior encephalocele (Figure 2); on the abdomen: hepatosplenomegaly with ascites; on the limbs: polydactyly (Figure 3) on the 4 distal segments, club feet (Figure 4); cleft palate (Figure 5); examination of the spine and external genital organs was normal. Autopsy examination was not approved by the family. Faced with this poly-malformative syndrome, the diagnosis of Meckel syndrome was suggested.
Discussion
Dysencephalia Splanchnocystica or MKS, all synonyms of the same syndrome due the etymology of the Meckel Gruber Syndrome. In 1822 this syndrome was first described by Johann Friedrich Meckel, a German anatomist, who found two twins who died with identical malformations: occipital encephalocele, polydactyly, and polycystic kidneys [3]. Then, in 1934 George B Gruber described familial cases with the same clinical features, including omphalocele, genital ambiguity and hepatic fibrosis; it was he who proposed the term splacnocystic dysencephaly to these findings [3]. It was Opitz and Howe who proposed the name Meckel-Gruber syndrome with subsequent refinement of the diagnostic criteria [3,4].
Meckel-Gruber syndrome is a lethal, autosomal recessive syndrome; the risk of recurrence is 25% with each pregnancy. It has been frequently described in consanguineous marriages, but also occurs in nonconsanguineous couples. It has a penetrance of 100% and variable expressivity. A wide variety of malformations have been observed in this syndrome, the most consistent being occipital encephalocele, polycystic kidneys and polydactyly [3,4].
The worldwide incidence varies between 1 per 13 250 - 140 000 live births. A high incidence has been reported in the Indian Guajarati population with 1 birth in 3,500 (carrier rate of 1 in 18). There is either a predilection for the population of Finland, where the incidence at birth is 1/9,000. Meckel syndrome is estimated to account for 5% of all neural tube defects in Finland. In Belgian ancestry, the incidence is 1/3,000 persons. The incidence of MKS is also high in consanguineous groups such as the Bedouin tribes of Kuwait (1 in 3,530 live births) [7] and the population of Saudi Arabia (1 in 3,500 live births) [8]. It usually occurs in the context of consanguineous unions; more than 200 cases have been reported in the literature [5,6].
Meckel-Gruber syndrome is caused by mutations in genes encoding proteins that are structural or functional components of primary cilia. The diseases caused by mutations in ciliary genes are collectively called ciliopathies, and the MKS is the most severe of this group of diseases. It can be caused by mutations in any one of at least eight genes; CEP290, B9D1, B9D2, CC2D2A, MKS1, RPGRIP1L, TMEM216, TMEM67. It is known or suspected that the proteins produced by these genes play a role in cellular structures called cilia. Cilia are involved in signaling pathways that transmit information between cells. They are important for the structure and function of many cell types, including brain cells and certain cells in the kidney and liver. Mutations in genes associated with Meckel’s syndrome cause problems with cilia structure and function. Defects in these cellular structures can disrupt chemical signaling pathways important during early development.
MKS is highly phenotypically variable, highly genetically heterogeneous, and antagonistic to other related ciliopathies, such as Joubert syndrome, which greatly complicates diagnosis. Recent advances in genetic engineering have greatly improved diagnosis, genetic counseling, and clinical management of MKS families through the extensive use of multigene panels for molecular testing, unfortunately, these advances in scientific research are not yet accessible.
Autosomal recessive (AR) inheritance of MKS is confirmed by equal occurrence in males and females, concordance in monozygotic twins, examples of affected siblings, and possibly parental consanguinity. If the disease occurred during a previous pregnancy, the risk of recurrence is 25%, Hence the importance of genetic counseling for this couple. In all cases it is necessary to perform a family genealogical tree of the couple for genetic counseling [9]. MKS is fatal in utero or shortly after birth, often due to pulmonary hypoplasia, although there have been exceptional reports of survival [9].
Prenatal diagnosis, in our institution, is fundamentally ultrasound, in this case it occurred at 14 weeks of gestation, which began with abnormal findings in routine standard studies that led to a more detailed study for diagnosis, although it has been described that it could be diagnosed as early as 11-12 weeks, especially in families at high risk through the visualization of anomalies in the central nervous system, kidneys and even in the fingers.
The diagnostic criteria for prenatal Meckel syndrome were based on finding the presence of two of the three classic malformations: occipital meningo-encephalocele, multicystic renal dysplasia and bilateral postaxial polydactyly which are described to be present in 100% of this case [10]; another constant malformation is hepatic fibrosis which could not be established. These findings are thus considered as major criteria for diagnosis [11,12]. Besides hepatic fibrosis, all diagnostic criteria can be assessed by ultrasound diagnosis during the 1st trimester of pregnancy, thus underlining the importance of prenatal diagnosis in this syndrome, given the difficulty of access to genetic diagnosis. Transabdominal ultrasound performed at 10-14 weeks gestation for polycystic kidney disease (after 9 weeks of gestation), occipital encephalocele (after 13 weeks of gestation), and polydactyly (after 11 weeks of gestation), occurring in both high-risk and low-risk pregnancies [9]. Visualization can be affected by oligohydramnios, but is less problematic when performed in the first trimester of pregnancy [13]. Transvaginal scan allows further investigation of abnormalities. The fetal bladder can be seen by ultrasound from week 11 of gestation, and the absence of an apparent fetal bladder frequently indicates renal dysfunction.
Malformations at the central nervous system are variable; defects of the corpus callosum and of the midline in general, hydrocephalus have been described. Posterior fossa dysgenesis (posterior fossa cysts, vermis agenesis, etc.) is also frequent. In all cases postnatal anatomopathological examination is recommended to reach the diagnosis, but this was not possible in this case due to the parent’s decision. There are multiple other malformations described that should be searched in this syndrome, although their frequency is lower, for example, cleft palate (45% of cases) present in this case, cardiac malformations (20%), tongue, splenic malformations, ocular (microphthalmia, retinal dysplasia, congenital cataract, etc.), facial, micrognathia, low-implantation ears [3,14].
Fetal MRI is an alternative if ultrasound is inconclusive or lack of amniotic fluid prevents providing clear images. It can provide better soft-tissue resolution than ultrasound but is rarely performed before 18 weeks’ gestation. the transabdominal or vaginal endoscopy in the first trimester of pregnancy allows the diagnosis by visualization of the surface anatomy of the fetus. Fetoscopy allows direct observation of polydactyly and occipital encephalocele from 11 weeks of gestational age [15]. Prenatal diagnosis is also possible by a combination of these techniques, α-fetoprotein testing of amniotic fluid, and DNA testing of the fetus and parents. For example, elevated maternal α-fetoprotein levels during prenatal screening may be associated with this syndrome [16].
The differential diagnosis should be made with chromosomopathies such as trisomy 13, trisomy 18, in both of which a karyotype would make a diagnosis; with Smith- Lemli-Opitz syndrome, Bardet-Biedl syndrome, Beemer-Langer syndrome, Carpenter-Hunter syndrome, autosomal recessive but genetically heterogeneous Joubert syndrome, which is frequently associated with renal dysplasia and polydactyly, and many other polymalformative syndromes. Diagnostic orientation will be occipital encephalocele, polycystic kidneys, polydactyly and other findings that will be confirmed by autopsy.
Conclusion
The syndrome is a rare disease of autosomal recessive inheritance, of higher frequency in consanguineous couples, although it also occurs in non-consanguineous marriages; the diagnosis, in our environment, is mainly based on ultrasound scan, it has been described that it can be performed from the 11th week in patients with known risk and through genetic diagnosis. Genetic counseling is important since there is a risk of recurrence of 25% in each pregnancy, this will allow the couple to decide on their obstetric future.
To Know more about Global Journal of Reproductive Medicine
Click here: https://juniperpublishers.com/gjorm/index.php
To Know more about our Juniper Publishers
Click here: https://juniperpublishers.com/index.php





No comments:
Post a Comment