Academic Journal of Polymer Science - Juniper Publishers
Abstract
The purpose of this study was to evaluate the effect of adding various concentrations of castor oil (2% and 4% v/v) on the properties of edible films based on carboxymethyl cellulose (CMC). Moisture content, water solubility, tensile strength, elongation at break, elastic modulus, water vapor permeability, optical and thermal properties and antioxidant activity of the films were examined. The results demonstrated that the presence of castor oil led to a decrease in moisture content, water solubility and mechanical strength of the films. The film containing 4% of oil showed the highest water vapor permeability. The optical properties measurement represented that all of the samples were transparent. By the addition of castor oil, the antioxidant activity of the films improved largely. Thermal properties of the samples had also been investigated and it was determined that the effect of castor oil on the melting point was negligible. Finally, the performance of the coatings for protecting fresh apples was studied with measuring the pH value of apples and performing some field test.
Introduction
Every year a large portion of foods, especially fruits and vegetables, from the time they are harvested until they reach to consumers, go through different kinds of qualitative and quantitative deterioration and loss [1]. Various factors are involved in food deterioration such as microorganisms, mechanical damages, time and light. Therefore, if we fail to protect the food, it can be damaged within hours or days [2]. Currently, there are numerous technologies used for the purpose of fruits and food preservation. Some include controlled atmosphere storage and modified atmosphere packaging, innovative osmotic dehydration technologies, electro-osmotic dewatering , thermal pasteurization, and gaseous ozone and ozonated water treatments [3,4]. One way to prevent or delay the food deterioration is the use of edible coatings. During the past decade, edible coating has been widely used for this purpose [5-9]. Some advantages of edible coatings include extending shelf-life of fresh fruits, reducing amount of waste produced from packaging processes, improving appearance and fulfilling environmental safety, as well as enhancing nutritional properties due to containing lots of beneficial biopolymers and biological compounds including polysaccharides, proteins, and lipids. Moreover, they decrease water loss and retard ripening of fruits due to barrier mechanism, and are also able to enhance antioxidant properties of fruits and vegetables [10-13].
Any material used to cover foods in order to enhance the shelf life, which may be eaten with the food, is an edible coating [2]. Various studies have been conducted on the properties and applications of edible films and coatings [14-18]. The initial studies on edible coatings can be traced back to 1967, where Hardenburg studied the application of wax used by Chinese on citrus fruits [19]. Since 1986 there were various studies on edible coating including wax emulsions, oils, cellulose, chitin and chitosan, and their impact on diverse properties of fruits and food like shelf-life [20-26]. However, more research is needed because there are no edible films that can be used for all purposes. As other technologies, edible coatings can also bring some challenges like undesirable tastes caused by presence of wax materials, hydrophilic nature of edible coatings which makes them moisture-sensitive and limits their moisture-barrier performance, poor adhesion and coverage, and insufficient mechanical properties of biopolymers comparing to the synthetic ones [27,28]. In general, an ideal edible film must have characteristics such as being nontoxic, allergenic or indigestible materials, protecting foods from mechanical damages, good adhesion, maintaining the appearance of the product and having an easy and economical production [2]. Proteins, carbohydrates and lipids are the main film forming agents in edible coatings [29]. Generally, lipids will reduce water transmission in edible coatings, polysaccharides are good for controlling the transmission of gases and protein-based films have proper mechanical strength [29]. These materials can be used either individually or as a blend (composite films) in edible coatings [29].
Carboxymethyl cellulose (CMC) is a conventional polysaccharide, which is one of the common derivatives of cellulose. CMC is an anionic polysaccharide, which unlike cellulose is soluble in water [30]. CMC has wide variety of uses in different industries such as foods and coatings [31] and therefore is an excellent choice for edible coatings because it has no toxic or allergic effects. It is one of the most desirable polysaccharide polymers due to its excellent properties including perfect film forming ability, availability, low price and high viscosity, which can be used to produce both edible and degradable films and coatings [31]. In most cases, a plasticizer must be added to the film forming solution in order to reduce the brittleness of CMC films. However, using plasticizers will affect the mechanical and permeability properties of the films [32]. The most common food grade plasticizers are some polyols including glycerol, mannitol, sorbitol, and sucrose [33]. Glycerol is a clear, colorless and odorless liquid, which is soluble in water due to its hydrogen bonds. This component reduces the film's fragility by being located between the CMC chains (enhancing chain mobility) and also by absorbing water [34]. The beneficial properties of some lipids, including their good compatibility with other film constituents and good barrier properties against water vapor and other gases, make them an ideal choice for edible coatings and films [35,36]. Lipid compounds that are commonly used to make films and edible coatings include edible oils, fatty acids and waxes [37]. The efficiency of lipid used in edible films and coatings depends on the nature of the lipid, particularly its structure, hydrophobicity and its interaction with other components in the system [2]. Castor oil is a viscous and non-volatile liquid with a pale yellow color. High amount of both resinoleic acid (RA) and double bonds results in oxidative stability of the castor oil as well as long shelf life [37]. The antioxidant activity of castor oil also makes it an excellent choice for use in edible coatings in order to prevent food degradation.
In this study, the effect of castor oil on mechanical, optical, physical and thermal properties of CMC-based edible films along with their antioxidant activity have been investigated. The results showed potential use for castor oil in edible coatings.
Materials and Methods
Materials
Carboxymethyl Cellulose (CMC) with purity of 99.6% and viscosity of 2787 cps ; Castor oil with density of 0.959 g/mL [38] and Polysorbate 80 (Tween 80) were purchased from Pasargad Novin chemical Co. Glycerol (USP grade) was purchased from PALMAC. Analytical grade of Ascorbic acid was obtained from Merck (Darmstadt, Germany). CMC is a white, granule-shaped powder used for increasing the viscosity. Castor oil consists of various fatty acids mostly containing ricinoleic acid (12-hydroxyoctadecenoic acid) [39]. Tween 80 which is a highly viscous liquid, is used as a surfactant [40,41].
Preparation of films
Film forming solution was prepared by adding 1 g of CMC to 100 ml of distilled water (1% w/v). The system was under continuous agitation by magnet stirrer and the temperature was set on 75℃. After 40 minutes, a clear solution was achieved. Then 1 ml of Glycerol (as plasticizer) was added to the film forming solution and stirring went on for another 15 minutes. At this point, the film forming solution was ready in order to prepare the control films (films containing no castor oil). To do so, 25 g of the solution was poured in the middle of plastic circular plates (with 10 cm diameter and 1 cm height) and dried at room temperature in about 48 hours. Other film samples were prepared as described below:
a. CO-2: 0.2 ml of Tween 80 (proportional to castor oil) was added to the film forming solution under continuous stirring. After 15 minutes, 2 ml of castor oil was also added to the solution and the stirring continued for another 30 minutes.
b. CO-4: 0.4 ml of Tween 80 was added to the film forming solution under continuous stirring. After 15 minutes, 4 ml of castor oil was also added to the solution and the stirring continued for another 30 minutes.
c. AA1: 1 g of ascorbic acid was added to the film forming solution and the system was under stirring for 10 minutes.
d. AA2: 0.4 ml of Tween 80 was added to the film forming solution under continuous stirring. After 15 minutes, 4 ml of castor oil was also added to the solution and the stirring continued for another 30 minutes. Following that, 1 g of ascorbic acid was added to the solution and stirring continued for 10 minutes.
The procedure of preparing film from all of the mentioned solutions was similar to the control films. Also, to do some field tests and measuring pH the sample films were applied on apple by silicon brush. All the dried films were peeled of the plates and kept in a desiccator at 25℃ and 50% relative humidity (RH) for 72 hours until further evaluations.
Thickness
A hand-held micrometer was used to measure the film thickness. For each film, the thickness was measured in several areas (at least at five locations) and the final thickness was reported as the average value.
Optical properties
The color of each film was monitored using a calorimeter in the CIE Lab system. The films were placed on a standard white background (L*= 89.7584, a* = -0.5083 and b* = -2.0585) and their “Lab” values were determined. The color difference (ΔE) between the films and the standard background, whiteness index (WI) and yellowness index (YI) were calculated using the following equations:
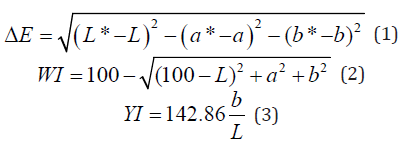
Where “L” value represents the lightness of the sample (varies from 0 (black) to 100 (white)), “a” represents the redness-greenness balance of the sample (from negative (green) to positive (red) values) and “b” indicates the yellowness-blueness balance of the film (between negative (blue) and positive (yellow) values) [37].
Moisture content (MC)
The moisture content of each film was determined based on weight loss. In this method, the film was first weighed and then placed inside the oven at 110°C. The weight of the film was measured every hour until it reached a constant value. Finally, the amount of moisture in the film was obtained from the difference between the initial and the final weight. The percentage of water in the film was calculated by the following equation:
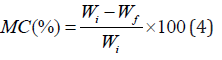
Where Wi is the weight of the film before being placed in the oven and Wf is the weight of the film after that. The test was repeated three times for each sample to secure a more accurate result.
Water solubility (WS)
The solubility of the film in water was evaluated based on the method described by Gontard et al. with some modifications [42]. The initial weight of the film was measured after drying the sample at 110 °C. Then the dry samples were immersed in 100 ml water for 5 hours at 25°C under continuous stirring. After filtration, to determine the final dry weight, the portion of the film, which was undissolved in water, was placed in an oven at 110°C to reach a constant weight. The water solubility percentage of the film was calculated by the following equation:
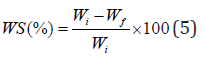
Where Wi is the initial dry weight and Wf is the final dry weight of the films (after immersing in water).
In order to obtain a consistent result, water solubility of each film was evaluated three times and the average value was reported.
Water vapor permeability (WVP)
The water vapor permeability of each film was determined based on the instructions provided by ASTM E96 [43]. Cups with an average diameter of 3.5 cm and a depth of 1 cm were used to determine WVP. The films were cut in circle shapes with slightly larger diameter than of the cup. After placing 3 g of water (RH = 100%) in each cup, they were covered with sample films. Each cup was placed in a desiccator containing silica gel (RH = 0%) and the temperature was maintained at 25°C. The cups were weighed every 24 hours (With a precision of 0.0001 g). The weight change of the cup was plotted as a function of time and the slope of this curve (weight change versus time) was calculated by a linear regression. Water vapor transmission rate (WVTR) was obtained by dividing this slope by the transmission area (m2). Finally, the WVP was evaluated from the following equation:
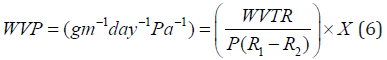
Where P is the saturated vapor pressure of water at test temperature (25℃), R1 is the RH inside the desiccator, R2 is the RH inside the cup and X is the film thickness (in meter).
Antioxidant activity
The antioxidant activity of the film samples was investigated through DPPH radical scavenging activity based on instruction of Brand-Williams et al. [44] with some modification. This test is based on the ability of the samples to donate hydrogen or electron and is evaluated by measuring the amount of color reduction of 2,2-diphenyl-1-picrylhydrazyl (DPPH) solution in methanol (a solution with purple hue). DPPH radical is absorbed at 517 nm but the amount of absorbance decreases when it is exposed to an antioxidant. To evaluate this property, 25 mg of the sample film was added to 5 ml of distilled water and the mixture was stirred continuously by a magnet Stirrer. Then 0.1 ml of the resulted solution was added to 3.9 ml of DPPH solution (0.1 mM methanol solution) and the sample was incubated in darkness for one hour at room temperature. The sample’s absorbance was then measured at 517 nm and the scavenging activity was calculated by the following equation:
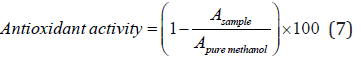
Where A is the absorbance at 517 nm.
Mechanical properties
Tensile strength, elongation at break and elastic modules of the films were measured by the universal testing machine. The test was conducted based on the ASTM D882-91 standard [45]. For this purpose, the films were cut into rectangle shapes (about 2 cm x 10 cm) and placed between the grips. The initial space between the grips and the crosshead speed were set at 50 mm and 10 mm/min, respectively (with 1 kg load cell). Mechanical properties were evaluated for three replications of each sample.
Thermal properties
The thermal properties of the films were investigated by DSC test, which is described below. Approximately 5 mg of the film was placed in an aluminum pan. The reference was also an empty aluminum pan. Thermal behavior of different films were measured at a thermal scanning rate of 5℃/min in three steps. First heating from 30℃ to 100°C, then cooling from 100℃ to 30°C and last heating again from 30℃ to 250°C. The melting point (Tm) of the films was determined in this process.
pH value
To evaluate the pH of apples, 10 g of each sample (with its coating) was added to 100 ml of distilled water and then stirred for 30 minutes. After filtering the mixture, its pH was measured and reported by a digital pH-Meter. In order to compare the results, the same process was performed on control apples (apples with no coating). The pH measurement was started immediately after applying coating on the apples and was repeated every 4 days. During the test process, apples were kept at room temperature.
Field test
To evaluate the performance of the coating, three apples were picked from a store and processed as described below:
a. The first apple was kept unwashed.
b. The second one was washed.
c. The last one was washed and then the CO-4 film forming solution was applied on it with a silicon brush.
All three samples were then kept at a dark place at room temperature for six months.
Statistical analysis
All the Data were analyzed using MATLAB and Statistics Toolbox Release R2018b, The MathWorks, Inc., Natick, Massachusetts, United States. The results were reported as mean ± standard deviation.
Results and Discussion
Thickness
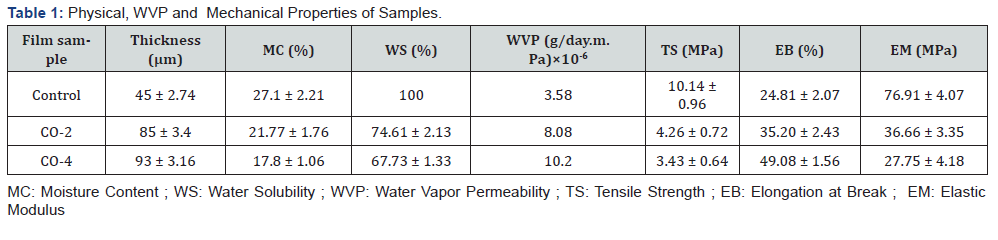
Thickness is an important parameter that affects some properties of the films such as transparency, permeability and mechanical strength [46]. The thickness of the films varied from 45 to 95 microns. As shown in Table 1, the thickness of the control film (without castor oil) was 45 microns and with increasing the oil content in the samples, their thickness increased until it reached 95 microns for the film containing 4% castor oil. The reason is that by adding oil to the film-forming solution, the solid content of the final coating increases, because castor oil enters the CMC matrix, which results in an increase in thickness. Same result have been reported by Shojaee-Aliabadi et al. [47].
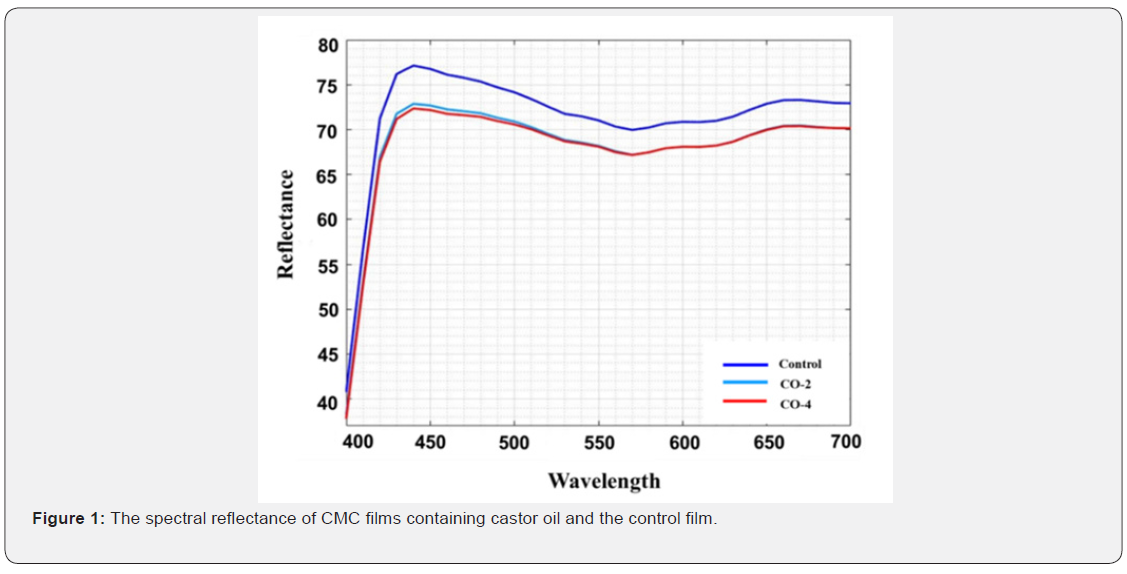
Optical properties
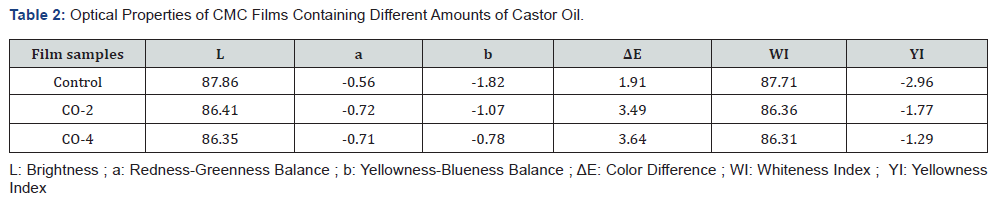
Optical properties or appearance is an important factor of edible coatings and films as they affect the acceptancy of a product by the consumers. The least expectation from a coating is to maintain the appearance of the product if cannot improve it. The CIE Lab, color difference (ΔE), whiteness index (WI) and yellowness index (YI) values used to describe the optical properties are given in Table 2. The spectral reflectance curves of the samples are also shown in Figure 1. As illustrated, the control film had the highest area under the curve and therefore the highest brightness. The L value (brightness) of the samples decreased slightly with increasing oil content. In addition, the yellowness index of the specimens increased marginally with increasing oil content in the film structure, which could be due to the absorption of light by castor oil at low wavelengths. For the same reason, the color difference of the samples increased moderately with the addition of oil to the film (the color difference between the CO-2 and CO-4 samples having different oil content was not significant). Finally, the whiteness index of the samples decreased slightly with the addition of oil to the system. The presence of castor oil and increasing the amount of it reduced the transparency of the film to some negligible degree. The reason is that by adding oil to the film forming solution, which is a water-insoluble fraction, the system becomes an emulsion, resulting in a decrease in the transparency of the film relative to the oil-free sample [48]. Overall, the addition of castor oil to CMC films had no major negative effects on their appearance.
MC
Moisture content of films is an important factor in determining coatings quality. High moisture content can provide an ideal environment for the growth of microorganisms and cause deterioration during long storage periods. The MC also helps edible films to melt in mouth when eaten [2]. The MC of each specimen is reported in Table 1. As can be seen, samples containing castor oil had a lower moisture content than the control film, and the MC decreases with increasing oil content from 2% to 4%. The reason for this decrease can be mainly attributed to the increased hydrophobicity of the films due to the increased oil content. In addition to increasing hydrophobicity, the interaction between castor oil and the hydroxyl groups present in the CMC impedes water absorption by these groups, thereby reducing the amount of water in the film. Similar results were found by previous studies [47,49].
WS
Moisture content of a film can also affect its solubility in water. The higher the moisture content of the film, the greater the solubility in water. The water solubility of each film sample is reported in Table 1. As shown in the diagram, the CMC film containing no castor oil (control sample) is completely soluble in water. The solubility in water decreased with increasing castor oil concentration in samples due to the high amount of hydrophobicity in the film matrix and preventing CMC hydroxyl groups from absorbing water. The lowest water solubility was observed in CO-4 sample containing 4% castor oil in its structure.
WVP
WVP is an important factor to investigate the performance of edible films and coatings and can be influenced by characteristics such as film integrity, hydrophobic ratio and film thickness [50]. Knowing WVP of the film is very helpful for preventing the mass transfer from food to the surrounding environment and therefore extending the shelf life of the product. The values of G/t, which is the slope of the weight change curve over time of the samples, are shown in Figure 2. Using these slopes, the WVP values were calculated for the samples (Table 1). It is observed that with increasing oil content, the slope of the curve increased, resulting in a higher WVP. The film containing 4% oil shows the highest amount of WVP. While the common expectation is that increasing oil content would decrease the permeability of the samples due to the increased hydrophobicity of the films, the exact opposite result has been achieved. This could be due to the fact that the increase in castor oil concentration in CMC films, besides increasing the hydrophobicity ratio, had a negative effect on the cohesion of the film matrix. The weakening of the cohesion forces in the matrix accelerates the transfer phenomenon and ultimately increased the water vapor permeability. Furthermore, castor oil also has a softening effect similar to glycerol. This makes the movement of polymer chains in the film easier, which increases permeability. Similar result was observed in the work of Dashipour et al. [49].
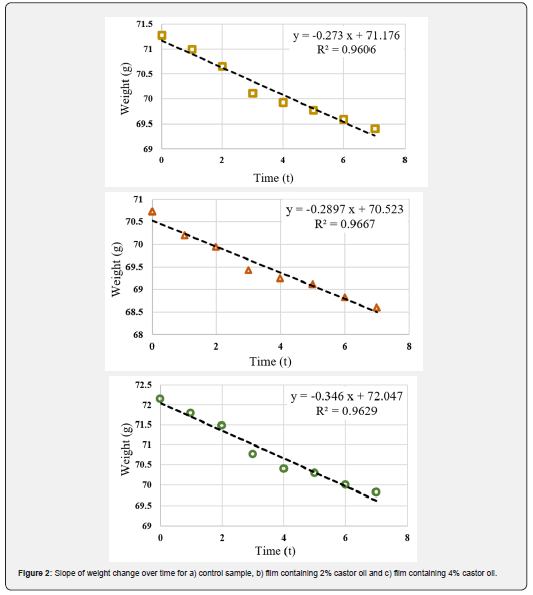
Antioxidant activity
The ability of an edible coating to prevent foods from oxidation is an important factor in determining the coating's performance to extend the shelf life of the product. The higher the antioxidant activity of the coating, the better its function in protecting the food. In this study, the antioxidant activity of the samples was measured by DPPH scavenging activity method, and the results are shown in Figure 3. As expected, the control film showed no antioxidant activity, as there is no compound in its structure capable of radical absorption. The amount of antioxidant activity in oil-containing films is significantly higher than that of control sample. By increasing the oil concentration from 2% to 4%, the scavenging activity of the film also increased and reached 46.3%, which is desirable. The antioxidant activity of films containing just castor oil was lower than that of ascorbic acid containing samples. The highest scavenging activity was for AA2 sample, which had both castor oil and ascorbic acid (95.4%). The radical scavenging activity of the oil-containing specimens results from the unsaturated double bands present in castor oil structure. These double bands become saturated by absorbing free radicals; therefore, prevent the product from oxidizing.
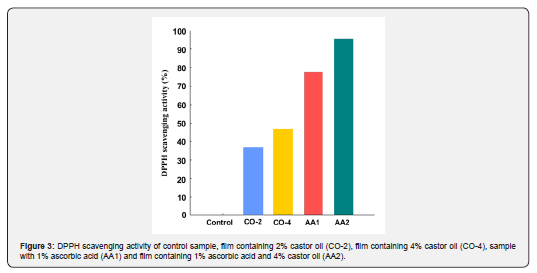
Mechanical properties
Characteristics of edible films such as tensile strength (TS), elastic modulus (EM) and elongation at break (EB) are very important in coatings and packaging and can be helpful to predict coatings performance [51]. Table 1 shows the TS, EM and EB values of samples. As illustrated, the tensile strength of the control sample was 10.14 MPa, which decreased significantly with increasing oil content and reached 3.43 MPa in CO-4 film. Similarly, the elastic modulus of the films decreased with increasing castor oil from 76.91 MPa to 27.75 MPa. On the other hand, with increasing castor oil concentration, the flexibility of the films improved and therefore their elongation at break increased from 24.81% for control sample to 49.08% for CO-4 sample. This can be due to the fact that the addition of castor oil created a heterogeneous film, which results in decreasing mechanical strength and increasing flexibility [52]. In addition, as mentioned earlier, castor oil has some level of softening property. Therefore, its molecules can be placed between CMC chains and facilitate their movements and as a result increased the films’ flexibility, desirably.
Thermal properties
Determination of thermal properties, especially glass transition temperature, is very effective in evaluating the performance of edible films and coatings. If the glass transition temperature of the film is much higher than the ambient temperature, the film would be very brittle, but it would have low permeability. However, if the Tg is lower than the ambient temperature, the permeability would be very high and the film would be very soft and flexible, which will not provide the proper mechanical protection for the product. As a result, if the glass transition temperature of the films is slightly above ambient temperature (close to ambient temperature), it will be in a desirable range of permeability and mechanical strength [46]. Therefore, if castor oil could reduce the Tg and Tm of the specimens, it will be considered a beneficial effect. Thermal diagram of the samples obtained by performing DSC experiment and is shown in Figure 4. As shown in the diagrams, in the temperature range of 208℃ to 211°C there are large and endothermic peaks, which could be attributed to the melting temperature of the CMC matrix. The Tm of the control sample is 210.99°C, and with increasing castor oil content to 4% (CO-4), the Tm reached 208.39°C. As presented, no glass transition temperature was observed in the samples due to the low sensitivity of the test equipment.
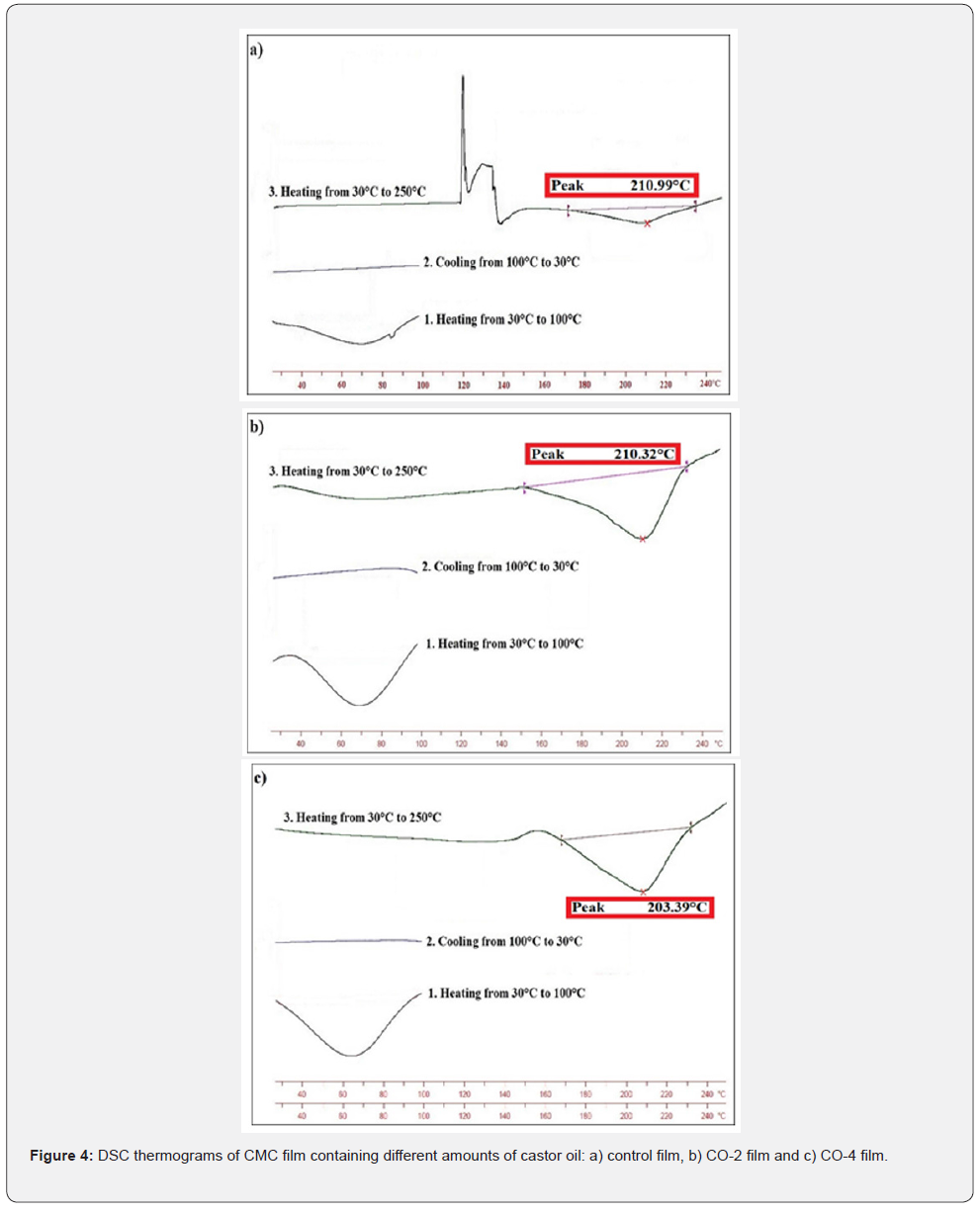
pH value
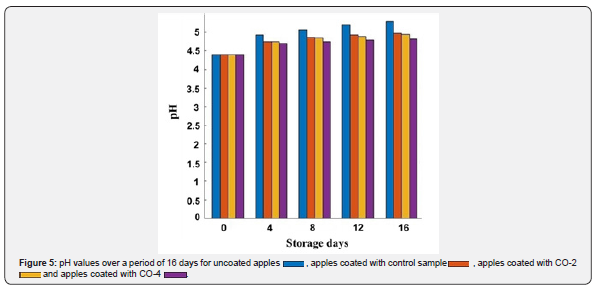
The microbial growth in the food produces nitrogen-containing compounds that increase the pH of the product [53]. The coating should prevent this increase in pH by restraining the microbial growth. The better the coating performs in this matter, the lower the pH increases and the longer the shelf life of the product will be. The pH values of control and coated apples were measured over 16 days, which are represented in Figure 5. As can be seen, the highest pH increase was for uncoated apples and the lowest increase was for apples with CO-4 coating. In general, coatings (especially the ones with castor oil) were successful in preventing pH increase and their pH was lower than non-coated apples after 16 days. This result shows that CMC-based coatings containing castor oil prevented pH value from increasing.
Field test
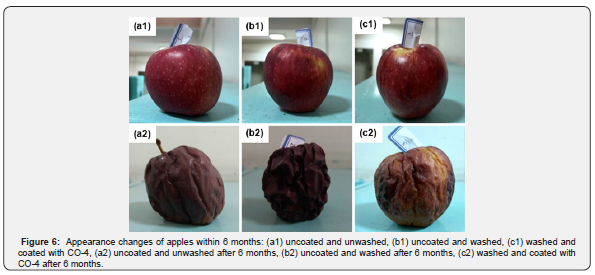
As mentioned earlier, one of the causes of food deterioration is time. Nowadays, the process of preparing foods until they are ready for consumers’ use takes a considerable amount of time. During this time-consuming process, foods could undergo water loss and deterioration. Therefore, the performance of edible coatings over time is very important. Figure 6 shows the changes of apples after being exposed to ambient temperature for 6 months. Although the coated apple (Figure 6, c2) was also suffered from high deterioration, it had less water loss and discoloration than the other two apples, which had no coating. The coated apple also had a much better physical state and was more firm and less wrinkled than the other two.
Conclusion
The presence of castor oil in the film forming solution increased the thickness of the resulting CMC films, but due to its hydrophobic nature, reduced the moisture content and water solubility of the films. The addition of castor oil in carboxymethyl cellulose films resulted in higher permeability due to the weakening of cohesion forces in CMC matrix and softening properties of the oil. The mechanical strength of the films was also weakened for the same reason. As the oil content increased, the color difference and yellowness index of the films increased, and the whiteness index decreased slightly. Due to the presence of unsaturated double bonds in the castor oil structure, this oil had the ability of radical absorption and therefore antioxidant activity. Addition of castor oil had no significant effect on the thermal properties of the carboxymethyl cellulose films and reduced the melting temperature of the samples by 3°C. The coatings prevented the early deterioration of apples and reduced the pH increasing rate by preventing the growth and activity of the microorganisms.
To Know more about Academic Journal of Polymer Science
Click here: https://juniperpublishers.com/index.php




No comments:
Post a Comment