Open Access Journal of Surgery - Juniper Publishers
Abstract
Corynebacterium striatum is a Gram-positive bacillus and too potentially pathogenic microorganism with the ability to produce nosocomial outbreaks. Additionally, C. striatum has been associated with an increasing number of invasive infections as such as: sepsis, endocarditis, meningitis, osteomyelitis. However, there are a few studies focused on virulence factors that may contribute to elucidate the mechanisms concerned about healthcare associated infections by Corynebacterium spp. including C. striatum. The relevance of biofilm formation to development of nosocomial infections was recognized and the effects of antimicrobial agents on these surface-attached communities remain under investigation. Therefore, the biofilm formation by Corynebacterium striatum were validated quantitatively conform previous methodology. Additionally, o was analyzed by electron scanning microscopy of biofilm formation on abiotic substrates. Therefore, were used four different clones isolated in nosocomial outbreak in University Hospital in Rio de Janeiro city. The biofilm formation analysis was performed by CFU quantification and SEM according to previously described to the surface of glass and polyurethane, glass slides and catheters fragments were inoculated by immersion in 106 CFU.ml-1 bacterial suspension in Trypticase Soy Broth and incubated to 37ºC/48h. To quantitative evaluation, the formed biofilm was then extracted by abrasion and quantified by CFU count.
To structural analysis, sections of glass coverslips and polyurethane catheters were fixed in 2.5% glutaraldehyde, post- fixed in 1% osmium tetroxide solution and dehydrated an ethanol gradient. Subsequently catheter segments were submitted to critical point drying with carbon dioxide, covered with 10nm gold layer, and examined with a JEOL JSM 5310 scanning electron microscope. The results revealed C. striatum ability to adhere to hydrophilic (glass) and hydrophobic polyurethane, abiotic surfaces at different intensities. Additionally, C. striatum strains showed biofilm formation in the polyurethane catheter surface 48h post-incubation and maturation of the biofilm resulting in the generation of a complex architecture with channels and pores that formed their three-dimensional structure (Figure 2 C,D,E), the presence of extracellular matrix. Conclusion: All samples the of C. striatum tested adhere on substrates tested at different intensities and your complex structure has several characteristics that show the present of mature biofilm. Discussion: From these results, effective and appropriate measures should be taken to control this the hospital environment and thus to decrease the incidence of outbreaks caused by C. striatum.
Keywords: Antimicrobial multidrug resistance; Bacteremia; Biofilm; Catheter-related infection; C. striatum; Nosocomial outbreak, Surgical wards.
Abbreviations: CFU: colony-forming unit; CLSI: Clinical da Laboratory Standards Institute; CVC: central venous catheter; HMJ: Hospital Municipal de Jesus; HUPE: Hospital Universitário Pedro Ernesto; ICU: intensive care unit; MDR: multidrug-resistant; MDS: multidrug-susceptible; MIC: minimum inhibitory concentration; MLSB: macrolides, lincosamides and streptogramins B; PFGE: pulsed-field gel electrophoresis; QRDR: quinolone-resistance determinant region; UERJ: Universidade do Estado do Rio de Janeiro; UPGMA: unweighted-pair group method using average linkages.
Introduction
Corynebacterium spp. is widely disseminated in the environment and can colonize the skin and mucous membranes of humans as part of the normal microbiota [1]. Because of these characteristics, as well as challenges in its identification, Corynebacterium spp. remain frequently considered as contaminants in clinical microbiological laboratories and by health professionals in many countries. Multidrug-resistant (MDR) and multi-susceptible (MDS) C. striatum strains have been reported with increased frequency as a pathogen of severe nosocomial infections and outbreaks in both industrialized and developing countries prolonged duration of hospitalization, advanced stage of chronic obstructive pulmonary disease, recent administration of antibiotics and exposure to an invasive diagnostic procedure have been highlighted as commonly found risk factors for acquiring MDR C. striatum infections [2]. Empirical antibiotic therapy may select MDR Gram-positive skin flora that may become the etiologic agent of nosocomial invasive diseases [2]. The emergence of MDR C. striatum and its involvement in nosocomial infections require appropriate interpretive criteria to the selection of the adequate antibiotic therapy [3] (Figure 1).
Studies have evidenced C. striatum as an emerging multidrug-resistant (MDR) pathogen related to nosocomial outbreaks in several countries [3]. Nosocomial spread of MDR C. striatum strains, especially in long-term hospitalized patients with prolonged exposure to broad-spectrum antibiotics and admitted in intensive care units (ICU) and surgical wards using continuous and prolonged medical devices and/or respiratory recuperation [4]. The ability of C. striatum survival and biofilm formation on abiotic surface of varied types, demonstrated that including indwelling medical devices may facilitate colonization and infection by C. striatum [4,5]. Therefore, the pathogenicity of C. striatum should not be underestimated and the virulence factors that support C. striatum infections related to the health service (HAIs) need further investigation. For all these reasons, it was considered a priority to establish more reliable methods to identify Corynebacterium clinical isolates at the species level [6]. Accordingly, the pathogenicity of C. striatum should not be underestimated and the virulence factors that support C. striatum infections related to the health service (HAIs) need further investigation.
In Brazilian tertiary hospital located in Rio de Janeiro metropolitan areas were documented a nosocomial outbreak caused by C. striatum. PFGE analysis indicated the presence of four PFGE profiles, including two related clones of MDR strains (PFGE I and II). The results of these studies demonstrate the predominance of PFGE-type I MDR isolates that are mainly isolated from ICUs and surgical wards [7]. C. striatum strains have largely been isolated in pure culture from tracheal aspirates of patients undergoing endotracheal intubation procedures. Other studies were conducted to evaluate the main factors that favor the spread of C. striatum in HUPE. Therefore, in the present study, we aimed to investigate the clonal relationship, antimicrobial susceptibility profiles and ability of biofilm formation on different abiotic surfaces, of analysis by electron scanning microscopy different MDR and MDS Corynebacterium striatum strains.
Methods
Bacterial strains
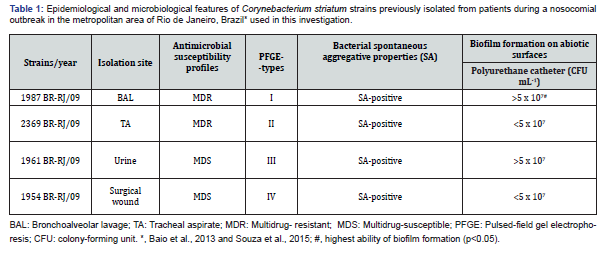
Table 1 shows the epidemiological and microbiological features of the partially studied C. striatum strains used in this investigation while C. striatum strains. Were four partially studied samples [7]. C. striatum isolated from hospitalized patients at University Hospital Pedro Ernesto state, Rio de Janeiro, Brazil. Microorganisms were stocked in-Skim Milk to - 70o C in storage center of Diphtheria Laboratory and Corynebacterioides of Clinical Importance - LDCIC - Department of Microbiology and Immunology - FCM / UERJ. Samples of C. striatum were previously identified phenotypically by conventional biochemical methods and the REF20900 API Coryne system (BioMérieux TM), following the manufacturer’s directions.
Resistant profile of MDR C. striatum
Susceptibility testing: Antimicrobial susceptibility profiles were determined by the disk diffusion method in cation-adjusted Mueller-Hinton agar supplemented with 5% sheep blood. Breakpoints for the susceptible strains were used as suggested by the Clinical Laboratory Standards Institute for bacteria excluded from tables 2A-K. As there is not yet a defined standard for interpreting these results, the standard proposed in CLSI document M45-A (ISBN 1-56238-607-7) was used (CLSI 2017) [8]. The breakpoints for S. aureus were considered in the cases of penicillin, oxacillin, and ampicillin. For the other antimicrobial agents, we used the breakpoints for other microorganisms, but not Haemophilus spp. or Neisseria gonorrhoeae, which had been validated by previous studies. Intermediate results were considered resistant. The antibiotics (Oxoid SA, Spain) tested included penicillin (10 U), ampicillin (30 μg), methicillin (5μg), cefotaxime (30 μg), cefepime (30 μg), ceftriaxone (30μg), imipenem (10 μg), erythromycin (15 μg), clindamycin (2μg), linezolid (30 μg), ciprofloxacin (5 μg), moxifloxocin (5 μg), tetracycline (30 μg), gentamicin (10 μg), rifampin (5 μg), fosfomycin (200 μg), vancomycin (30 μg), mupirocin (200 μg), tobramycin (10 μg), nitrofurantoin (300 μg) and ticarcillin/ clavulanate (75 μg/10 μg). C. striatum strains presenting resistance to more than three different classes of antibiotics were classified as MDR as previously defined by Magiorakos et al. [8].
Quantitative and qualitative analyses of biofilm formation on different abiotic surfaces
Investigation was performed using the following abiotic substrates lodged into 24-well flat-bottomed polystyrene microtiter plates: (i) 13 mm glass coverslips (Sigma-Aldrich), (ii) sterile 0.5 cm segments of polyurethane catheters (16-gauge percutaneous nephrostomy catheters Intracath; Deseret Pharmaceutical Co, USA) (iii) 13mm thermanox coverslips (NUNCTM) and (iv) sterile 0.5cm segments of metal parts of catheters (16-gauge percutaneous nephrostomy catheters Intracath; Deseret Pharmaceutical Co, USA). The quantification of bacteria associated to biofilm was determined by biofilm sand abrasion and culture as previously described by Souza and co- workers (2015) [4,5]. Briefly, each abiotic substrate was incubated with 108 CFU mL-1 of bacterial suspension that was mixed with 500μL TSB and incubated for 48h at 37ºC, to allow biofilm formation. Additionally, the biofilm formation was evaluated by SEM. Briefly, sections of polyurethane catheters were fixed in 2.5% glutaraldehyde, post-fixed in 1% osmium tetroxide and dehydrated in a graded series of ethanol. Subsequently, materials segments biofilms submitted tested were subjected to critical point drying with carbon dioxide, covered with a 10 nm layer of gold palladium, and examined with a JEOL JSM 5310 scanning electron microscope. Sterile polyurethane catheters (negative control) were also processed by SEM directly upon removal from commercial packaging [4,5].
Statistical analysis
Each experiment was carried out in triplicate and repeated three times. Student’s t test was used to compare means of experiments; p<0.05 and/or p<0.001 were considered statistically significant.
Results
Epidemiological and microbiological features of C. striatum strains previously isolated from patients of a University hospital at the metropolitan area of Rio de Janeiro, Brazil used in this study (Table 1) showed MDR profiles for strains 1987/I – BAL and 2369/II - tracheal aspirate that were susceptible only to vancomycin, linezolid and tetracycline. Two other strains (1954/IV surgical wound isolate and 1961/III urine isolate) were susceptible to most of the tested drugs (MDS) except mupirocin, fosfomycin and ticarcillin/clavulanate.
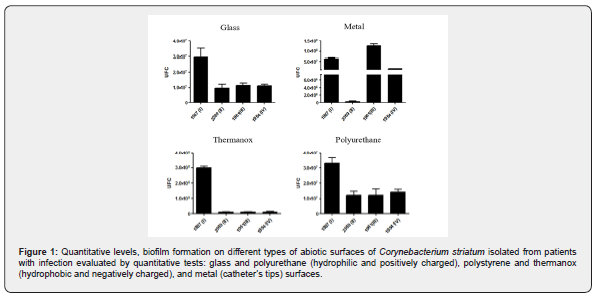
Quantitative analyses biofilm produces:
Data displayed in Figure 2 indicated that all four MDR and MDS C. striatum strains of different PFGE-types were found capable to produce mature biofilm (48h) on steel surface in addition to abiotic surfaces of glass, polyurethane, and polystyrene, but at different levels. Biofilms produced on these abiotic hydrophilic and positively charged (steel, glass, and polyurethane) and hydrophobic and negatively charged (polystyrene). Experiments with MDS 1961/III (urine) followed by MDR 1987/I (BAL) strains showed higher number of viable sessile bacterial cells recovered from biofilm formation a steel surfaces while MDR 1987/I strain showed a higher number of viable sessile bacterial cells recovered from biofilm formation on glass, polyurethane, and especially polystyrene surfaces. Lowest ability formation on all abiotic surfaces tested was observed for both 2369/II (tracheal aspirate) and 1954/IV (surgical wound), independent of antimicrobial susceptibility profiles (Figure 2).
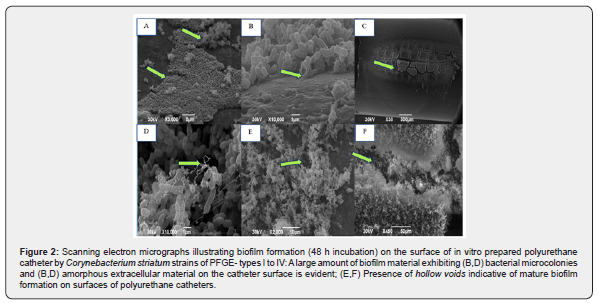
Morphological aspects of biofilm formation on polyurethane and silicone catheters evaluated by SEM:
Micrographs illustrating biofilm formation on the surface of polyurethane and silicone catheters by C. striatum 1987/PFGE profile I (Figure 2) and 2369/PFGE profile II (Figure 2) isolates demonstrated by SEM are displayed in Figure 2 showed micro colony formation (a hallmark of biofilm formation) by auto aggregative C. striatum on polyurethane surface. SEM assays also evidenced the presence of hollow voids, and extracellular matrix indicative of mature biofilm formation on surfaces of polyurethane (Figure 2) and silicone (Figure 2) catheters.
Discussion
Over the last decades, the proliferation of antibiotic-resistant pathogens has been a growing problem, in both industrialized and developing countries. Present data indicate bloodstream and catheter-related infections due to different clones of MDR. C. striatum in Brazil. These findings emphasized that C. striatum cultivated from blood and catheter segments should not be considered only as contaminant [6-11], since in our study most of the isolates were found in pure cultures (82%) or in significant numbers. The use of indwelling medical devices (e.g., central venous catheters) in current therapeutic practice is associated with 80-90% of hospital-acquired bloodstream and deep tissue infections. New knowledge in the pathogenesis of catheter-related bloodstream infections may lead to advances in the prevention and management of these infections. In the Brazilian hospital investigated in this study, 43% of C. striatum were isolated from catheter segments [5,9].
It has been estimated that 80% of human bacterial infections are biofilm-associated. Biofilms increase the cost of medical assistance and extend hospitalization [12-14]. More effective biofilm control strategies should result as researchers develop more reliable techniques for measuring biofilms and antimicrobial- drug resistance as well as better model systems for evaluating control strategies [5,9,12]. In the present study, biofilm formation and survival on five abiotic surfaces were demonstrated 48 h post-infection of bacterial cells representative of MDR C. striatum PFGE profiles I and II isolated from patients with bloodstream infections, but at different levels: glass, metal, polyurethane, and silicone. Equally to C. striatum PFGE profile I isolated from patients undergoing endotracheal intubation procedures, PFGE profile I isolated from bloodstream and catheter-related infections also showed a higher ability to adhere to and to survive on abiotic surfaces of medical devices including those used in invasive procedures. MDR C. striatum viable cells were able to multiply and to produce mature biofilms on both types of catheter surfaces. The results indicate the presence of features that contribute to the presence of this opportunistic pathogen in a hospital environment and its ability to form biofilm adhering onto various surfaces, thus facilitating their presence in various materials for hospital use as, respirators, catheters, and others. Health and epidemiological teams should be aware adopting measures to correct the isolation and identification of this microorganism, as well as direct the use of the most effective disinfectants, are essential to reducing the incidence of C. striatum in hospital. The virulent capacity of C. striatum should not be underestimated, particularly among high-risk patients. Therefore, antimicrobial susceptibility testing should be performed on clinically significant C. striatum isolates. Medical surveillance programs should include control strategies to decrease potential risk factors of nosocomial infections and outbreaks due to C. striatum.
To Know more about Open Access Journal of Surgery
Click here: https://juniperpublishers.com/index.php




No comments:
Post a Comment