JOJ Sciences - Juniper Publishers
Abstract
Potato (Solanum tuberosum L.) today is the fifth most significant crop worldwide after wheat, maize, rice and sugar cane. Conventionally intogressing agronomic traits in potato is considered laborious and time consuming task because of sexuality barriers between wild and cultivated potatoes. However, potato has gone through genetic manipulations with the advent of genetic engineering technologies. These technologies have helped the researchers to introduce traits of economic importance. Several studies for abiotic (i.e. drought, chilling, heat, salt) tolerances and improvement in nutrient quality have been documented. Modern day technologies have emerged as a necessary tool in potato breeding programs, strengthening classical strategies to improve yield and yield contributing factors. The present review article describes the genetic improvements in potato by scientists worldwide utilizing modern biotechnological approaches to enahnce abiotic tolerance in crop along with the future prospects of the transgenic potato.
Keywords: Genetic improvements; Abiotic stress; GMOs
Abbreviations: GNP: Gross National Agricultural Product; BV: Biological Value; IPM: Integrated Pest Management; ABA: Abscisic Acid; bZIP: Basic Leucine Zipper; TF: Transcription Factor; CE: GC-rich Coupling Elements; ABRE: ABA-responsive Element; CBP80: Cap-Binding Protein 80; ROS: Reactive Oxygen Species; BADH: Betaine Aldehyde Dehydrogenase; COD: Choline Oxidase; NT: Non-transgenic; TPS1: Trehalose-6-phosphate Synthase; amiRNAs: Artificial MicroRNAs; DREB; Dehydration-responsive Element-binding, DRE/CRT: Dehydration-responsive Element/C-repeat; EMSA: Electrophoretic Mobility Shift Assay; P5CS: 𝛿1-pyrroline-5-carboxylate Synthetase; CDPKs: Calcium-dependent Protein Kinases; GalUR: D-Galacturonic Acid Reductase; desA: Acyl-lipid 12-desaturase; SCOF-1: Soybean Cold Inducible Zinc Finger Transcription Factor; sHsps: Small Heat Shock Proteins; ATP: Adenosine Triphosphate; CuZnSOD: Copper–zinc Superoxide Dismutase; APX: Ascorbate Peroxidase; NDPK2: Plant Nucleoside Diphosphate Kinase 2; SSA: Transgenics with CuZnSOD genes and APX transgenes only; SN: Transgenics with NDPK2 only; 2-Cys Prx : 2-cysteine peroxiredoxin; MV: Methyl Viologen; DHN4: Dehydrin 4; miRNA: Micro RNA; CRISPR: Clustered Regularly Interspaced Short Palindromic Repeats; Cas9: CRISPR-associated protein9 nuclease
Introduction
Potato is a family of the Solanaceae, an enormous plant family with more than 3000 varieties [1]. More than 374,463,885 million tones of potatoes are produced worldwide [2]. As the global population reaches to 9.7 billion by 2050, the potato will play important role in securing global food resources. In Turkey, potato accounts for 3% of the gross national agricultural product (GNP), whereas it is about 3.1% in the EU-27 countries, hence contributing significantly to Turkish economy. It was cultivated on an area of 172,000 ha with the production of 3.9 million tons of potatoes. Central Anatolia including Nigde shares more than 60% potato production in this regard [3]. Potato has high amount of protein after soybean; patatin is the dominant storage protein [4]. The tubers of potato contain important dietary origin of starch, protein, vitamins and antioxidants [5]. Potato protein contains great biological value (BV) with a BV of 90-100 in contrast with whole egg (100) [6].
Potato crop is exposed to various sorts of abiotic stresses including drought saline cold and heat stresses. In areas where there is erratic rain fall or inadequate water supply, the cultivation of potato remains challenging [7]. As the world is going to face global warming issues, water restriction would be a threat in coming decades [8,9]. Drought stress periods may vary yearly in terms of duration and severity [10]. As per Hijmans [11], the potato losses due to climate change are expected to be 18-32% in first quarter of this century. Therefore, the development of transgenic drought resistant potatoes is an important issue. Potato as a frost-sensitive species adopts cool environment, and its regular growth and improvement are prevented by high temperature. When the temperature increases above 25 °C, tuber’s growth is terminated, and when the temperature rises to 39 °C, growth of stems and leaves are terminated [12]. Therefore, efforts are going on to develop abiotic stress tolerant potatoes. The potato presents unique challenges and advantages to plant breeders. As it is propagated vegetatively by tuber cuttings, potato cultivars don’t need to be bred to produce homogenous plants from true seed. A major disadvantage of potatoes for breeders is that potato is tetraploid, making it difficult to transfer desirable traits between cultivars and to have them expressed in subsequent progeny. The several species of Solanum are cultivated in Peru and Central America. These species provide a rich source for potential traits to breed into S. tuberosum, including tuber qualities (e.g. colors ranging from white to deep purple skin and flesh) and resistance to insect pests and diseases. Unfortunately, many of these wild Solanum relatives are diploid that further complicates breeding process. Hence, insertion of candidate gene(s) encoding for desirable economic trait by genetic engineering is a particularly attractive and valuable process for developing new potato cultivars [13]. There are many success reports of development of transgenic crops with traits of tolerance to abiotic stress [14]. These transgenic plants are becoming important components of integrated pest management (IPM) worldwide [15].
Improvement of Drought Stress Tolerance in Potatoes
S. tuberosum L. has been reported to be extremely sensitive to the drought stress, and with its leaves being more sensitive as this plant has less capability to absorb water from soil as compared to other crops [16]. The shallow root architectural system in potatoes is particularly detrimental to potatoes in that under drought, increased salinity and extreme temperature fluctuations, tuber yield and quality can be significantly plummeted [16-19]. Drought alone is expected to significantly decrease potato yield as much as 18 to 32% over the period of 2040-2069 [11]. Drought stress can bring about reduction in photosynthetic rate as well as reduction on biomass fresh weights of potato leaves and also it affects leaf number, leaf size and shoot length [16-18, 20-22]. One of the strategies that have been employed in ameliorating drought tolerance has been by introducing transgene that codes for bZIP (basic leucine zipper) TFs (transcription factors). On ABA (Abscisic acid) signal perception by specific receptors, signal transduction takes place when phosphorylation of bZIP TFs takes place via Ser/ Thr kinases which activate these bZIP TFs to bind to CE (GC-rich coupling elements) or cis-acting ABRE (ABA-responsive element) sequences to elicit various abiotic stresses [23]. Moon et al. [24] reported that the CaBZ1 gene isolated from hot pepper (Capsicum annuum) that encodes for bZIP transcription factor induced by ABA and under salinity and osmotic stresses when over-expressed in potatoes didn’t result in detrimental phenotypic traits in transgenic potatoes.
Under ABA treatment and drought condition, the transgenic plants showed a prompt stomatal closure with a decline in water loss rate and elevated yield under drought stress as compared to the wild-type. Kim et al. [25] generated improved drought tolerant transgenic potatoes based on decrease in water loss by overexpressing AtYUC6 gene associated with auxin biosynthesis in potato under CaMV 35S promoter using pCAMBIA1300pt–YUC6 construct. The transformed plants exhibited phenotypes associated with high-auxin like increment in the heights, erect stature, longevity and narrow-downward curled leaves. Pieczynski et al. [26] silenced the cap-binding protein 80 (CBP80) gene in potato cultivar Desiree using artificial miRNAs. CBP80 protein regulates miR159, MYB33 and MYB101 levels which are known to be the important regulators of ABA transduction pathway and drought tolerance. Downregulation of CBP80 was observed in silenced potato plants with decreased miR159 levels and increased levels of its target mRNAs, MYB33 and MYB101, which rendered higher drought tolerance in silenced plants. In addition, ABA-hypersensitive stomatal closing, elevation in thrichome and leaf stomata densities with decrease in number of michrochannels were reported, which correlated with increased tolerance to water stress. Similar pattern was reported in cbp80 mutant Arabidopsis. One of the major osmolytes, glycine betaine, accrues when encountered by abiotic stresses such as drought, high salinity and extreme temperature conditions. Not all plant species are capable of natural production or accretion of glycine beta. There has been extensive research with the ability to generate this compound in transgenics to ameliorate its tolerance to salt, cold, drought or high temperature stresses. Choline monoxygenase catalyzes conversion of choline to betaine aldehyde and ultimately to glycine beta under the catalysis of betaine aldehyde dehydrogenase [27-29]. Cheng et al. [30] cloned codA gene, obtained from Arthrobacter globiformis that directly converts choline to glycine beta, under the SWPA2 promoter to generate transgenic potatoes. The water stress was simulated with 20% PEG under the control of SWPA2 promoter. Accumulated glycine beta was reported in transgenic potatoes while non-transgenic potatoes exhibited no glycine beta accumulation as potato is not a glycine beta accumulating plant. Furthermore, reactive oxygen species (ROS) was shown to be accumulated and the transgenic potatoes exhibited strong drought resistance and recover ability. They further demonstrated that the glycine beta produced as a result of codA gene expression in transgenics could prevent membrane lipid peroxidation and degradation of chlorophyll caused by stress. Zhang et al. [31] reported that the cloning of transgene BADH gene (Betaine aldehyde dehydrogenase), under the control of drought- and NaCl- induced promoter rd29A from A. thaliana, into potato cultivar Gannongshu 2 increased the plant height of transgenic potatoes under NaCl and PEG stresses by 0.4 to 0.9cm, whereas fresh weight per plant increased from 17-29% as compared to non-transgenic controls indicating the drought and salt tolerances enhanced in the transgenics when BADH activity was upregulated.
Choline can be directly catalyzed to GB by choline oxidase (Cod) but it does not exist in higher plants. Different sources were adopted to transform codA gene: one by taking the gene from rhizobacterium and using promoter SWPA2 from sweet potato [32], and the other from transit peptide of Rubisco from tobacco [33]. The transgenic plants showed better tolerance to drought and salinity stress as compared to non-transgenic (NT) ones. There was also improvement in biomass of potted transgenic plants as compared to NTs when they were kept in water less conditions for 14 days to observe drought tolerance. Cheng et al. [30] further proceeded with these experiments by focusing on antioxidant system in drought conditions. The plants were kept in drought stress for 4 days and later were subject to rehydration conditions for 2 days. Transgenic plants were better to non-transgenic ones in many aspects including higher chlorophyll content, higher activity of antioxidant enzymes (caralase, peroxidases and superoxide dismutase), lower MDA and better recovery from water deficiency. Thus, tolerance against drought stress can be controlled in potato plants by increasing accumulation of GB. One of the elements involved in ROS signaling pathway in Arabidopsis is Arabidopsis annexin 1 (AtANN1). A family of calcium and membrane binding proteins, annexin, has also been found to confer stress tolerance to plants. ROS triggers boost in Ca2+ under salinity stress conditions and AtANN1 has been thought to be involved in activation of calcium conductance by NADPH oxidases in root epidermal cells. Annexin’s major role is to offset oxidative stress to maintain cell redox homeostasis and ameliorate tolerance against drought stress [34, 35]. Szalonek et al. [35] studied the role of StANN1 by overexpressing StANN1 in potatoes under CaMV 35S promoter and obtained more drought tolerant transgenics than the wild type with more water in green tissues, maintained chloroplast function with chlorophyll b and xanthophylls accretion. Also, the study inferred that the transgenic potatoes maintained effective photosynthesis during drought stress that increased its yield rate than non-transformed plants even under water stress. Trehalose overexpression has been shown to confer resistance against abiotic stress in plants such as rice and Arabidopsis [36-38]. However, in potatoes, it has been reported to be challenging to develop drought resistance transformants [39]. Nevertheless, improved drought tolerance in potatoes has been reported. Stiller et al. [40] cloned trehalose-6-phosphate synthase (TPS1) gene isolated from yeast with StDS2 drought inducible promoter and transformed the construct in White Lady potato cultivar. The resulting transgenics were drought tolerant and displayed higher stomatal conductance with increased net photosynthesis rates as compared to the wild types. There has also been a report on the use of artificial microRNAs (amiRNAs) in silencing CBP80/ABH1 gene in S. tuberosum to increase tolerance to water shortage conditions [41]. Myrothamnus falbellifolia (a terrestrial plant) and plants of Liliaceae family have been reported to synthesize glucosyl glycerol under drought stress conditions [42,43]. Transgenic potato plants were prepared by expressing ggpPS gene using 2 different types of promoters, i.e. CaMV35 and rd29A. Both type of transformed plants showed improvement in shoot length under both drought and saline stresses in green house conditions. Also, accumulation of GG was observed in leaves in both cases. But only rd29A transformants were able to accumulate GG in tuber [44]. Drought tolerance was introduced in potato plants by over expression of AtYUC6, as YUCCA family is known for its contribution in auxin biosynthesis. Potted transgenic plants of 4 months were evaluated, and they showed drought tolerance [25]. El-Banna et al. [45] used overexpression of gene regulating PR-10a (which is induced in osmotic/salinity stress) to control osmotic and salinity stress in potato callus. High proline accumulation and low oxidized glutathione was observed in transgenic callus in stress condition. Transgenic potatoes were developed by transferring sweet potato orange gene (ibOr). The transgenic plants were given drought stress in green house conditions. The transgenic lines showed improved resistances [46]. Thus, multiple researchers quoted above prove that there are a large number of genes which have important contribution for development of drought tolerance in potato plants.
Improvement of Salt Stress Tolerance in Potatoes
There are various implications of excessive soil salinity such as reduced yield and water potential, toxicity, alteration in metabolism of plants, ion imbalances and a drop-in assimilation of CO2. By the mid 21st century, a rise in arable land salinization seems to result in a loss of 50% of the arable land [47,48]. Potato has been categorized under moderately salt-sensitive crop; nonetheless, different potato cultivars respond differentially to salinity [49,50]. It has been identified that the Dehydration-responsive elementbinding (DREB)-1 TFs have been strongly induced under heat, drought, and high salinity as well as low-temperature stress conditions [51]. It has been elucidated that DREB1A and DREB2A TFs recognize DRE core sequence G/ACCGAC [52,53]. Additionally, DREB1A/CBF3 and DREB2A TFs specifically interact with cisacting dehydration-responsive element/C-repeat (DRE/CRT) region which play the role in drought and cold stress–responsive gene expression in A. thaliana [54]. Also, DREB1A/CBF1, DREB1A/ CBF3, DREB1B/CBF1 and DREB1C/CBF2 TFs have been found to be induced under low temperature stress conditions [55-57]. Bouaziz et al. [19] isolated StDREB2 gene from potato (cv. Nicola) plants and overexpressed it in transgenic potatoes, bioinformatics analysis of which unraveled that the StDREB2 protein belongs to the A-5 group of DREB subfamily. The overexpression in transgenic plants resulted in increased tolerance against salt stress. With the help of electrophoretic mobility shift assay (EMSA), they further found that this transcription factor was bound specifically to the DRE core element (ACCGAGA) in vitro. 𝛿1-pyrroline-5-carboxylate synthetase (P5CS) was increased in transgenics under salinity stress with collateral upregulation in proline accumulation inferring that StDREB2 might be responsive to abiotic stresses via ABA signaling regulation and through proline synthesis mechanism. In another similar study conducted by Bouaziz et al. [58], StDREB1 was found to be induced by drought, sodium chloride, cold temperature, and ABA. StDREB1 cDNA overexpression using pMDC32:StDREB1 construction in transgenic potatoes showed enhanced salt and drought stress tolerance in comparison to the control plants. As described above for StDREB2, the authors indicated that this increased stress tolerance could be due to P5CS-RNA expression as well the resulting proline osmoprotectant accumulation which also induced calcium-dependent protein kinases (CDPKs) stress responsive genes in standard and salt-stressed transgenics (Table 1).
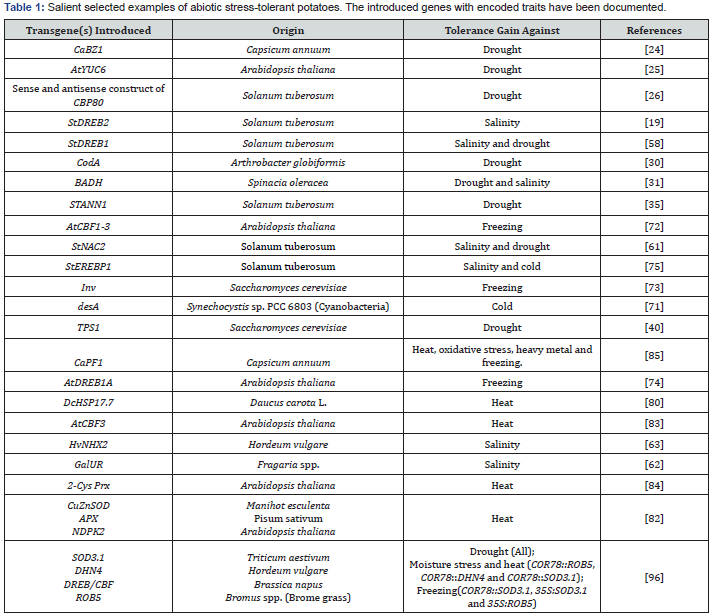
Agrobacterium mediated transformation was used to transform DREB1A of Arabidopsis in Longshu3 (L3) cultivar of potato. Positive gene integration and over expression of gene was confirmed using PCR, Southern blotting and semi quantitative RT-PCR Analysis. The transgenic lines showed very partial wilting when water was withheld for 14 days as compared to non-transgenic plants, thereby confirming that over expression of DREB1A improved drought tolerance in potato plants [59]. In plants, the other TF group, NAC, might be involved in regulating transcriptional reprogramming associated with plant stress responses and is involved in stress responses in plants [60]. StNAC2 overexpression under the control of CaMV 35S promoter in transgenic potato yielded ameliorated tolerance to salt in vitro and drought tolerance in pot growing condition. Phytophthora infestans also induced StNAC2 expression in addition to its induction by salt, drought and wounding stresses, indicating the possibility of cross-talking of signaling molecules involved in biotic and abiotic stresses [61].
Potato cultivar Taedong valley was overexpressed using Gal- UR gene (D-Galacturonic acid reductase) isolated from strawberry under the control of CaMV 35S promoter and exhibited enhanced ascorbate (AsA). The study identified the function of GSH (Reduced glutathione), its regulation via ascorbate pathway enzymes and its role in improving salt tolerance in plants. GalUR gene in transgenics enhanced ascorbic acid content (L-AsA), and ultimate< ly reduced oxidative stress-induced damage leading to salinity tolerance improvement with tub erization even at 200mM of NaCl in transformants as compared to wild types [62]. HvNHX2, an Na+/ H+ antiporter gene from barley, under the control of CaMV 35S was transformed to potato cultivars Skoroplodny and Jubilei Zhukova which conferred improved tolerance to NaCl. At 200mM NaCl, Jubilei Zhukova-derived transgenics survived, whereas Skoroplodny couldn’t. The transgenic plant had enhanced dry weight, root length, and suppressed cell explansion than the non-transformants. Postassium was found to be elevated in the transgenic roots instead of sodium [63]. Plant cells are prone to salinity damages in two perspectives i.e. ionic stress and osmotic stress. Osmoregulation in plants by using osmoregulator substances, e.g. Mannitol, has been used to confer salinity tolerance in plants [64- 66]. Potato is among the plant species which do not accumulate mannitol naturally. Rahnama et al. [67] produced transgenic potatoes by taking the mannitol-1-phosphate dehydrogenase (mtlD) gene from E. coli Mt1D gene was proved to confer salinity tolerance to potato.
Improvement of cold stress tolerance in potatoes
The other stress that holds devastation for the cultivated potatoes is freezing temperature, and as potatoes being frost sensitive species, these plants are inept to thrive well under cold stress condition with maximum freezing tolerance threshold of -3 °C, both before and after exposure to freezing temperatures. Drop in temperature can bring about a plunge in various enzymatic activities with ice formation under low temperature, and ultimately dehydration in cytoplasm. There are some wild potatoes which are better frost tolerant than the cultivated species which could be potential genetic resource for its use in genetic improvement of cultivated potato crops [68,69]. It has been shown that the desaturase gene protein can be helpful in conferring cold resistant stress in plants [70]. Amiri et al. [71] demonstrated that the desA gene (acyl-lipid 12-desaturase) isolated from Synechocystis sp. PCC 6803 (cyanobacteria) transformed in potato lines could ameliorate the cold tolerance by varying lipid polyunsaturated fatty acid levels. Under cold stress, the study observed more sensitivity in control plants than those for desA gene introduced transgenic plants. The damage index in control plants was more at 7 °C in the control plants, whereas those for the three lines in transgenics were significantly reduced. Additionally, the study concluded that the desA protein has negative role on stem growth as the stem length in transformants reduced by nearly 60% than the control plants.
The S. tuberosum cv. Umatilla was overexpressed with AtCBF1-3 driven by CaMV 35S or a stress-inducible Arabidopsis promoter rd29A. Under CaMV 35S promoter, AtCBF1 and AtCBF3 increased freezing tolerance about 2 °C, whereas AtCBF2 transgenics couldn’t. Under rd29A promoter, transgenics exhibited same level of freezing tolerance in few hours, whereas CaMV 35S exhibited tolerance only under low temperature but not under freezing condition. Transgenics with AtCBF constitutive expression under freezing temperature exhibited smaller leaves, stunted growth, delay in flowering, and yield loss. Under the same freezing condition, transgenics driven by rd29A resulted in improvement of phenotype indicating use of stress inducible promoter to direct CBF transgene expression to ameliorate freezing tolerance in potatoes [72]. Pino et al. [69] further reported that the ectopic overexpression of AtCBF1 in frost-sensitive S. tuberosum and S. commersonii both induced COR gene expression devoid of cold stimulus and stimulated increase in tolerance against cold temperature of about 1-2 °C and 4 °C in transgenic S. tuberosum and S. commersonii respectively as compared to their wild types. The study highlighted that S. tuberosum has CBF regulated genes that can increase the freezing tolerance of plants grown at warm temperature. However, the authors suggest that there may be lack of additional cold-tolerant genes in potatoes that made it incapable for transgenic potatoes to increase chilling tolerance beyond those conferred by AtCBF1 overexpression as it was reported that the transgenic S. tuberosum showed no further gain in freezing stress tolerance under low temperature condition while S. commersonii could exhibit better acclimation to low temperature. Invertase gene was isolated from yeast and transformed in potato (cv. Desiree) under the control of B33 class 1 tuber-specific promoter. Invertase activity, content of sugar and cold tolerance were measured by MDA content in in vitro plants. Under controlled conditions in transgenic plants, invertase content and sugar content increased in leaves at 22 °C and MDA content enhanced as compared to the wild types. The authors have suggested that the invertase gene as a transgene might have conferred tolerance at chilling temperature apparently as a result of variation in sugar ratio [73].
Desiree cultivar of potato was transformed with the gene construct of A. thaliana derived AtDREB1A, the gene driven by the rd29A promoter from the same source, which showed enhanced freezing tolerance than the wild type. Also, in many of the transgenic lines, the authors observed recovery from freezing stress [74]. Improved salt tolerance at 75mM and cold tolerance was observed in transgenic potatoes (cv. Superior) when StEREBP1 was overexpressed under a constitutive CaMV 35S promoter inferring that StEREBP1 is another TF associated with abiotic stresses in plants. The yield in elite transformant improved by approximately 50% under cold stress. Also, it was observed that StEREBP1 binds to DRE/CRT and DCC cis-elements for its activity and microarray and RT-PCR techniques showed that many other stress responsive genes containing GCC box had been under the overexpression of StEREBP1 TF [75].
Increased cold stress resistance was induced in potato plants by transformation of soybean cold inducible zinc finger transcription factor (SCOF-1). The transgenic plants were kept under cold stress at 4 °C for 5 days. The expression of SCOF-1 correlated positively with the cold stress. The results showed that overexpression of SCOF-1 can efficiently increase the tolerance against freezing stress [76].
Improvement of Heat Stress Tolerance in Potatoes
Potato yield, mainly in the warm tropical region, is narrowed down by high temperature by hindering synthesis of starch in tubers [77]. The potato tuber growth optimal temperature is approximately 20 °C as potato is a cool-season crop [78]. The high temperature has been reported to cause build-up of glycoalkaloid in potatoes that alters the carbohydrate metabolism in tuber tissue leading to heat-induced damage of potato tubers [79]. Increased temperatures can also lead to a drop-in tuber dry matter accumulation. The tetraploidy of the cultivated potatoes makes potato genome more byzantine and high degree of sterility has precluded development of conventional breeding. This makes genetic engineering of potatoes to improve thermotolerance in potatoes inevitable [80]. Most small heat shock proteins (sHsps) are perceived only under heat stress in vegetative tissues and not under normal growth conditions. With increment in temperature up to 10-15 °C above the normal growth condition could actually be lethal to the organisms and eventually can induce heat shock response and stress tolerance in plants [81]. sHsps are adenosine triphosphate (ATP)-independent molecular chaperones which preclude irreversible aggregation or initiates correct refolding of the incorrectly folded or partially damaged proteins. In addition, many studies have reported incorporation of sHSP genes in ameliorating thermotolerance in various organisms [80]. Enhanced thermotolerance in the transgenic Desiree potatoes, probably the first one in potato, were obtained when the carrot gene that codes for heat shock protein (DcHSP17.7 gene) was transformed, regulated by CaMV 35S promoter. Under normal condition without stress, DcHSP17.7 expressed constitutively, though not in abundant amount. Transgenics showed ameliorated stability of the cellular membrane at increased temperature and prompt high tuber yield even at constant 29 °C stress when compared with the non-transformants. The study found that there was a good increase in percentages and dry weight of microtubes [80].
Transgenic potato cv. Atlantic which expressed cassava CuZn- SOD (Copper–zinc superoxide dismutase), pea APX (Ascorbate peroxidase) and Arabidopsis NDPK2 (Plant nucleoside diphosphate kinase 2) genes together under the control of stress-inducible SWPA2 promoter, all of these genes were shown to be ameliorating tolerance to high temperature stress and methyl viologen- induced oxidative stress. This transgenics (SSAN) showed enhanced tolerance to methyl viologen than the non-transgenics, SSA (transgenics with CuZnSOD genes and APX transgenes only) and SN (transgenics with NDPK2 only) plants. SSAN sprayed with 40μM methyl viologen resulted 53% less visible damage than SSA and 83% less than SN. Furthermore, high temperature tolerance as high as 42 °C was achieved in SSAN transgenics with only 6.2% reduction in photosynthesis rate than those grown at 25 °C, while this drop in rate was 50% for SN and 18% for SSA transgenics [82]. Dou et al. [83] isolated CBF3 gene from Arabidopsis which can be induced under cold stress, cloned under CaMV 35S promoter control as well as rd29A promoter and transformed the construct into the ‘luyin NO. 1’ potato cultivar. AtCBF3 could be expressed under heat stress even at the temperature higher than 40 °C. The accretion of O2 • − and H2O2 was declined in the transformants as compared to the non-transformants with increased D1 protein accumulation, net phososynthetic rate and maximal PSII photochemical efficiency in transgenics. The results inferred that the amelioration in heat stress tolerance was exhibited as a result of ectopic expression of AtCBF3 gene which enhanced photosynthesis and antioxidant defense. Nevertheless, HSP70 accumulation was lesser in transgenics than the wild types indicating HSP70 role was uninvolved in the pathway.
In another study, Kim et al. [84] overexpressed potato cultivar Atlantic with antioxidative enzyme 2-cysteine peroxiredoxin (2-Cys Prx) gene, which aids in eliminating peroxides and shields the photosynthetic membrane from oxidative damage, regulated by stress-inducible SWPA2 promoter or 35S promoter. The treatment with 3μM methyl viologen (MV) on both promoter-driven transgenics exhibited approximately 33% and 15% less damage than the wild type. Photosynthetic activity for SWPA2-promoter driven transgenics declined by 25% when 300μM MV was sprayed onto whole plants, whereas for non-transgenics it dropped further to 60%. SWPA2-driven transgenics could tolerate up to 42 °C.
Multiple tolerance against stresses including heat, oxidative stress, heavy metal and freezing was achieved in transgenic potatoes when ERF/AP2-type TF CaPF1 gene isolated from Capsicum annuum was cloned under the control of CaMV 35S promoter and the construct vector was transformed to Atlantic potato cultivar. However, the tuber formation in transgenics in vitro was severely impeded as compared with the wild-type [85].
Use of Transcription Factor for Introducing Tolerance Against Abiotic Stress in Potatoes
In addition to the genes directly related to different stresses, transcription factors also play a vital role in plants’ natural defense against various abiotic stresses. So, transformation of better transcription factor can be a good strategy to enhance tolerance of plants against various abiotic stresses [72,86,87]. Transgenic potatoes were developed in independent researches via transforming AtDREB/CBF gene. Different promoters were used in different researches i.e. rd29A [72,74,88,89] and 35S promoters [72,90]. The transgenic potato plant showed increased resistance against cold stress, salinity and drought tolerance [91]. Youm et al. [85] transformed a CaPF1 gene from Capsicum annuum in potato plants, this gene encodes the transcription factor AP2/ERF which has role in tolerance against cold stress and pathogens. The results showed an increased tolerance against drought, freezing, heat, heavy metal ions and oxidative stress in transgenic lines but tuber formation was retarded in these transformants as compared to non-transgenic plants. R2R3-type Myb TF has been reported to be involved in secondary metabolism and responding to biotic and abiotic stresses [92,93]. It is encoded by IbMybb1 gene. Cheng et al. [94] transformed this gene in potato plants which ultimately showed higher level of secondary metabolites (anthocynins, flavonoids and total phenols). These secondary metabolites are released in drought and UV-B ray stresses, thus the transformants showed better response under these stresses. Shin et al. [95] developed transgenic potato plants by transformation of StMyb1R-1 gene (a stress inducible gene) which encodes R-1 type MYB-like TF. The transgenic plants showed better response under drought stress.
Combination of Many Different Tolerances
Waterer et al. [96] developed potato cultivar Desiree by introducing transgenes under the control of 35S promoter or stress-inducible Arabidopsis COR78 promoter. Four types of transgenes were used: wheat mitochondrial MnSOD (SOD3.1), barley dehydrin 4 (DHN4), cold-inducible transcription factor DREB/ CBF from canola and stress-inducible brome grass derived ROB5 gene coding for LEA group 3-like protein. In total, six transgenics were used: COR78:: SOD3:1, COR78::DHN4, COR78::DREB/ CBF, COR78::ROB5, 35S::SOD and 35S::ROB5. Many of the transformed lines were reported to produce higher yield even at the significant drought stress. Under moisture stress, COR78::ROB5, COR78::DHN4 and COR78::SOD3.1 transgenics performed with higher yield with heat tolerance up to 44 °C. The tolerance was seen the highest for COR78::SOD3.1 transformants. At 10 °C, 35S::- SOD3.1 grew better than the non-transformants and COR78::- SOD3.1, 35S:SOD3.1 and 35S:ROB5 transgenics also showed improved tolerance against freezing stress.
StnsLTP1 is a potential gene from potato which was reported to be thermo-tolerant. Transgenic potato lines were developed which showed overexpression of this gene. The transgenic plants not only showed increased cell membrane integrity under stress conditions but also depicted increased antioxidant enzyme activity. The stress related genes (StAPX, StCAT, StSOD, StHsfA3, StHSP70 and StSHSP20) were also unregulated in transgenic lines [97].
Future prospective
Micro RNAs as a prospective candidate in improving abiotic stress tolerance in potato
Micro RNAs (miRNA) have been known to play an important role in various abiotic stress tolerance and some of them have been elucidated for transgenic plants. miR156 overexpression in Arabidopsis revealed enhanced tolerance to heat stress [98], constitutive overexpression of miR169 in tomato ameliorated tolerance against drought [99], miR319 overexpression in transgenic rice enhanced cold stress tolerance [100] and miR402 overexpression in Arabidopsis increased tolerance against drought, salinity and cold stresses in the transformants [82]. Similar type of miRNA or other miRNAs that have been explored to be involved in various abiotic stress tolerances can be studied by overexpression, silencing or other manipulations in potatoes which could improve the abiotic stress tolerance in cultivated potatoes.
CRISPR/Cas9, an emerging technology for improving abiotic stress tolerance in potato
A newly introduced technology Clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated protein9 nuclease (Cas9) is revolutionizing the genetic engineering concepts in plants. CRISPR has been successfully implemented in various organisms including plants. It was first utilized in model plants like Arabidopsis, tobacco, and with passage of time it has been used for making genetic transitions in crops like maize, soybean, sorghum, wheat, woody plants, etc. [101-108]. Osakabe et al. [109] have used CRISPR for dealing with drought stress in Arabidopsis plants and they have achieved some success. But authors suggested that more studies are needed in this respect. CRIPSR/Cas9 has been utilized to study genes related to abiotic tolerance in plants. Two glycosyltransferase genes, i.e. UGT79B2 and UBT79B3, are thought to be responsible for making Arabidopsis plants tolerant in case of drought cold and salt stress. When these genes were knocked down by using CRISPR/Cas9 the plants became more susceptible to these stresses [110]. Shi et al. [111] utilized CRISPR/ Cas9 for generating transgenic maize with improved drought tolerance. Thus, studies need to be done in potatoes by utilizing the efficacy of CRISPR/Cas9 to enhance abiotic stress resistance in crop.
To Know more about JOJ Sciences
Click here: https://juniperpublishers.com/index.php





No comments:
Post a Comment