Pharmacology & Clinical Research - Juniper Publishers
Abstract
The present study was aimed to evaluate the anti-inflammatory potential of Biofield Energy Healing (the Trivedi Effect®-Consciousness Energy Healing) on the test formulation in colon cancer cell line (HT-29). Each ingredient of the test formulation was divided into two parts, one part was denoted as the untreated test formulation and the other part was demarcated as the Biofield Energy Treated test formulation, which received Biofield Energy Healing Treatment by a renowned Biofield Energy Healer, Mr. Mahendra Kumar Trivedi. MTT assay showed that the test formulation was found safe and non-toxic upto 122 μg/mL in HT-29 cells with more than 78% cell viability. The level of interleukin-6 (IL-6) expression was significantly reduced by 34.09% and 59.41% (p≤0.001) at 3 and 15 μM, respectively compared to the vehicle control (VC) group under the stimulation of tumor necrosis factor - alpha (TNF-α). Moreover, IL-8 level was significantly suppressed in the Biofield Energy Treated test formulation by 57.09% and 42.88% at 0.1 and 3 μM, respectively compared with the VC group. However, the Biofield Energy Treated test formulation further substantial altered the level of interferon gamma compared to the VC group. The Trivedi Effect®-Consciousness Energy Healing significantly regulate the inflammatory condition after treatment with the test formulation in colon cancer cell line (HT-29). This experimental data suggested that the Biofield Treated test formulation can be utilized for many inflammatory disease conditions such as rheumatoid arthritis, multiple sclerosis, psoriasis, inflammatory bowel diseases, scleroderma, and type 1 diabetes mellitus.
Keywords: Biofield Energy Healing; The Trivedi Effect®; Inflammation; Colon cancer cell line (HT-29); Pro-inflammatory cytokines
Abbreviations: FBS: Fetal bovine serum; MTT: 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide; ELISA: Enzyme-linked immunosorbent assay; NCCAM: National Center for Complementary and Alternative Medicine; CAM: Complementary and Alternative Medicine
Introduction
The relation among of the gut health, micro biota, and cytokines have been well studies and reported in past two decades against various inflammatory bowel diseases (IBD) and associated mucosal inflammations. The cytokines play an important role in the mechanism in inflammation especially in Crohn’s disease and ulcerative colitis. The pathogenesis of IBD is not completely understood, but the role of cytokines in the intestinal immune system has significant impact in disruption of normal state of controlled gut inflammations [1,2]. Innate immune response is the major response in gut inflammation and its related diseases. Most of the cytokines are secreted by activated dendritic cells and the macrophages, which regulates the inflammatory response in gut inflammatory diseases. Once, these cytokines are secreted by antigen presenting cells, they triggers and differentiate various T cells by activating adaptive immune response. Gut inflammation dysregulates the T-cells, and manage the over-reactive and auto-reactive cells. T-cell regulation or its overproduction leads to the development of gut inflammatory diseases [3]. These cells along with various types of cytokines play a complex role in inflammatory gut diseases [4, 5]. Thus, there is the need of some novel formulation which alters the level of cytokines to improve the gut health. The present study was aimed to test the impact of the Biofield Energy Treated test formulation comprised of zinc chloride, ferrous sulphate, copper chloride (II-cupric), vitamin B6 (pyridoxine HCl), vitamin B12 (cyanocobalamin), magnesium (II) gluconate, and cholecalciferol (vit. D3) against the colon cytokines. The novel test formulation was treated with Biofield Energy Healing Treatment, as one of the best CAM approach with significant therapeutic outcomes. Biofield Energy Healing is one of the emerging frontiers aspect to CAM and various clinical approach has been used with significant results [6-9]. CAM therapies have been recommended by The National Center for Complementary/Alternative Medicine (NCCAM) and there therapies exist in various forms such as external qigong, Johrei, Reiki, therapeutic touch, yoga, Qi Gong, polarity therapy, Tai Chi, pranic healing, deep breathing, chiropractic/osteopathic manipulation, guided imagery, meditation, massage, homeopathy, hypnotherapy, progressive relaxation, acupressure, acupuncture, special diets, relaxation techniques, Rolfing structural integration, healing touch, movement therapy, pilates, mindfulness, Ayurvedic medicine, traditional Chinese herbs and medicines in biological systems both in vitro and in vivo. Human Biofield Energy has subtle energy that has the capacity to work in an effective manner [10] with its various clinical benefits [11]. This energy can be harness and transmit it into living and non-living things by the process of Biofield Energy Healing Treatment. Biofield Energy Treatment (the Trivedi Effect®- Consciousness Energy Healing Treatment) has been extensively studied with significant outcomes in the field of pharmaceuticals [12-14], nutraceuticals [15,16], metals and ceramics [17-19], microbiology [20-22], microbial genetics [23, 24], cancer research [25,26], livestock, agriculture science [27-29], improved bioavailability of many compounds [30-32], improved skin health [33, 34], improved properties of nutraceuticals [35, 36], improved overall bone health [37-39], human health and wellness. Thus, the study was planned on colon cytokines estimation that could significantly helped to improve the prevalence of gut inflammatory diseases using novel test formulation consisting of minerals such as Mg, Zn, Fe, Cu and vitamins including B6, B12, D3 in colon cancer cell line (HT-29).
Materials and Methods
Chemicals and Reagents
Antibiotics solution (Penicillin-Streptomycin) was purchased from HiMedia, India. 3-(4, 5-dimethyl-2-thiazolyl) 2, 5 diphenyl-2 H-tetrazolium) (MTT), Dulbecco’s Modified Eagle’s Medium (DMEM), NaHCO3, and EDTA were purchased from Sigma Chemical Corp. (St. Louis, MO), a subsidiary of Sigma-Aldrich Corporation. ELISA (enzyme-link immunosorbent assay) assay kits for all cytokines tumor necrosis factor alpha (TNF-α), macrophage inflammatory protein-1α (MIP-1α), and interleukin-1 beta (IL-1β) were purchased from R&D Systems, USA. Fetal bovine serum (FBS) was purchased from GIBCO, USA. Iron sulfate, copper chloride, and cholecalciferol (vitamin D3) were obtained from Sigma Chemical Co. (St. Louis, MO). Zinc chloride and magnesium (II) gluconate hydrate were obtained from TCI, Japan. Pyridoxine- HCL (vit-B6), cyanocobalamin (vit-B12) were procured from Alfa Aesar, USA. All other chemicals used in this study were analytical grade available in India.
Test Formulation and Reference Standard
The test formulation contained a combination of vitamins with minerals viz. iron sulfate, copper chloride, zinc chloride and magnesium (II) gluconate hydrate, cholecalciferol (vitamin D3), pyridoxine-HCL (Vit-B6), and cyanocobalamin (Vit-B12). Tumor necrosis factor alpha (TNF-α) was used as an inflammatory stimulant, while epigallocatechin-3-gallate (EGCG) was used as a reference standard (positive control) for immunomodulatory action in colon cancer cell line (HT-29).
Biofield Energy Healing Strategies
One part of each ingredient of the test formulation did not receive any sort of treatment and was defined as the untreated test formulation group, while another part received Biofield Energy Treatment known as Biofield Treated Test formulation by Mr. Mahendra Kumar Trivedi, a renowned Biofield Energy Healer under standard laboratory conditions for ~3 minutes. This treatment was provided through the Biofield Energy Healer unique Energy Transmission process (the Trivedi Effect®) to the test formulation. Further, the untreated test formulation was treated with a “sham” healer for comparison purposes. The “sham” healer did not have any knowledge about the Biofield Energy Treatment. After that, the Biofield Energy treated and untreated test formulations were kept in similar sealed conditions and used for the in vitro study on colon cancer cell line (HT-29) for cytokines estimation.
Experimental Design
The colon cancer cell line (HT-29) was divided into four different groups. Group 1 comprised of the HT-29 cells vehicle was denoted as the vehicle control, group 2 included cells with epigallocatechin-3-gallate (EGCG) as positive control at various concentrations. Group 3 and 4 included the cells with the untreated and Biofield Energy Treated test formulation, respectively at concentration range 0.1 to 15 μg/mL in presence of tumor necrosis factor - alpha (TNF-α).
Cytotoxicity by MTT Assay
The effect of the Biofield Energy Treated and untreated test formulations at a wide range concentration were tested for cell viability using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. The number of viable cells were determined by the ability of mitochondria to convert MTT to formazan dye. The details procedure was followed as per Plikerd et al. 2017 [40]. The effect of the test formulation on cell viability of HT-29 cells was determined as:
%Cell viability=100-%cytotoxicity (1)
Where; % cytotoxicity = [(O.D. of control cells – O.D. of cells treated with the test formulation)/O.D. of control cells]*100.
The results of the concentrations that showed >75% viability were selected subsequently for cytokine estimation.
Determination of Cytokine levels by ELISA
The HT-29 cell suspension in DMEM medium containing 10% FBS was plated at a density of 0.5 X 106 in 12-well cell culture plates. Cells were incubated at 37 °C for 24 hours. Cells were sera starved by replacing medium with DMEM containing 0% FBS and again incubated at 37 °C for another 24 hours. Cells were treated with proprietary test formulation at selected noncytotoxic concentrations and stimulated with Hu-TNF-α. Cells treated with TNF-α + EGCG were included as the positive control. After treatment, cells were incubated in a 5% CO2 incubator for 72 hours. After incubation, culture supernatants were collected from each well and stored at -20 °C until analysis. The level of cytokines (IL-6, IL-8, and IFN-γ) in culture supernatants of HT-29 cells were determined using ELISA as per manufacturer’s instructions.
Statistical Analysis
All the data were expressed as mean of three replicates ± SEM and were subjected to one-way analysis of variance (ANOVA) followed by Dunnett’s test and Student’s t-test for two groups comparison. Statistical significance was considered at p≤0.05.
Results & Discussion
MTT Assay
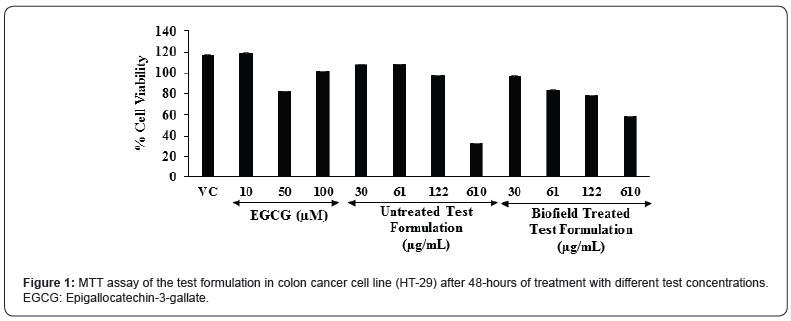
The cell viability results are summarized in the Figure 1. The percent cell viability in the vehicle control (VC) group was found as 117.5%. Moreover, the positive control, epigallocatechin-3- gallate (EGCG) showed 119.2%, 83%, and 101.2% cell viability at the concentration of 10, 50, and 100 μM, respectively. Further, the untreated test formulation showed more than 97% cell viability upto 122 μM; while the Biofield Treated test formulation showed more than 78% cell viability upto 122 μM. Based on the MTT cell viability assay the test formulation was found as safe and nontoxic upto the concentration of 122 μM. MTT assay is widely used for the cell toxicity against any test formulations. In addition, this assay was found as more rapid, less costly, less time consuming, and non-radioactive method as compared with the other assays. This assay display cell proliferation results on the basis of the cell growth and metabolic activity [41]. MTT assay suggest that the concentrations of the test formulation were found safe up to 122 μg/mL with respect to the viability in the colon cancer cell line (HT-29).
Estimation of Interleukin-6 (IL-6) Expression
The level of interleukin-6 (IL-6) expression in colon cancer (HT-29) cells is represented in the Figure 2. The positive control, epigallocatechin-3-gallate (EGCG) was significantly reduced the level of IL-6 by 38.71%, 92.43%, 91.74%, and 98.20% (p≤0.001) at the concentrations of 1, 10, 50, and 100 μM, respectively as compared to the vehicle control (VC) group. Moreover, the untreated test formulation showed significant reduction of IL-6 by 11.96%, 35.94%, and 52.3% (p≤0.001) at 0.1, 3, and 15 μM, respectively as compared to the VC group. Further, the Biofield Energy Treated test formulation group showed 14.19%, 34.09%, and 59.41% (p≤0.001) reduction of IL-6 at 0.1, 3, and 15 μM, respectively as compared to the VC group under the stimulation of TNF-α stimulation. Besides, the Biofield Treated test formulation also significantly reduced the level of IL-6 by 14.87% as compared to the untreated test formulation group. Overall, the minerals and vitamin-based Biofield Energy Treated test formulation showed an anti-inflammatory activity by reducing the level of IL-6 under the stimulation of TNF-α as compared with the vehicle control as well as untreated test formulation groups. Hence, the Biofield Energy Treated test formulation could be used a major role in immune-related disorders and also defined as controlling factor for many diseases [42]. Thus, it can be suggested that the Biofield Energy Treated test formulation can be used in many inflammatory disorders.

Estimation of IL-8 Expression
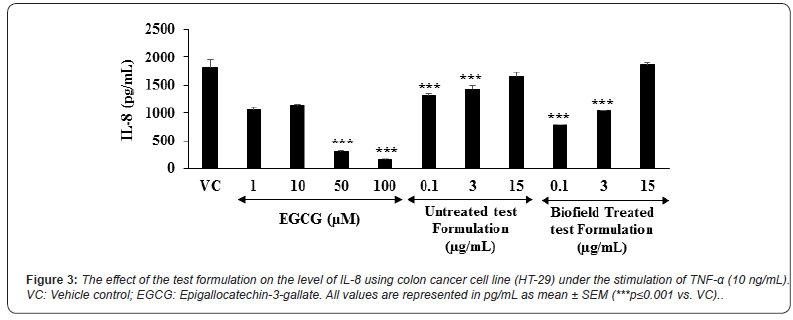
The level of interleukin-8 (IL-8) expression in colon cancer (HT-29) cells is represented in the Figure 3. The positive control, epigallocatechin-3-gallate (EGCG) was significantly reduced the level of IL-8 by 41.49%, 36.77%, 82.89% (p≤0.001), and 91.21% (p≤0.001) at the concentrations of 1,10,50, and 100 μM, respectively as compared to the vehicle control (VC) group. Moreover, the untreated test formulation showed significant reduction of IL-6 by 27.34% (p≤0.001), 21.03% ( ≤0.001), and 9.08% at 0.1, 3, and 15 μM, respectively as compared to the VC group. Further, the Biofield Energy Treated test formulation group showed significant (p≤0.001) reduction of IL-8 by 57.09% and 42.88% at 0.1 and 3 μM, respectively as compared to the VC group under TNF-α stimulation. Besides, the Biofield Treated test formulation also significantly reduced the level of IL-8 by 40.94% and 38.25% at 0.1 and 3 μg/mL, respectively as compared to the untreated test formulation group. Overall, the minerals and vitamin-based Biofield Energy Treated test formulation showed an anti-inflammatory activity by reducing the level of IL-8 under the stimulation of TNF-α as compared with the vehicle control as well as untreated test formulation group. Chronic inflammatory conditions leads to the massive production of proinflammatory factors such as chemokines. IL-8 is one of the chemokine in chronic inflammation and it is initially act as a neutrophil chemotactic and activating factor [43,44]. Overall, the experimental data suggested that Biofield Energy Healing Treatment has the significant capacity to reduce the level of IL-8 with respect to vehicle control and untreated test formulation.
Estimation of IFN-γ Expression
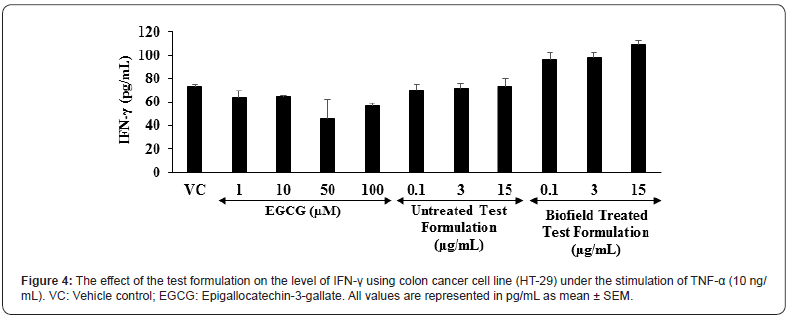
The level of interferon gamma (IFN-γ) expression in colon cancer (HT-29) cells is shown in the Figure 4. The positive control, epigallocatechin-3-gallate (EGCG) was significantly reduced the level of IFN-γ by 13.64%, 12.64%, 37.64%, and 22.99% at the concentrations of 1, 10, 50, and 100 μM, respectively as compared to the vehicle control (VC) group. Moreover, the untreated test formulation showed significant reduction of IFN-γ by 4.55% and 2.78% at 0.1 and 3 μM, respectively as compared to the VC group. Further, the Biofield Energy Treated test formulation significantly altered the level of IFN-γ as compared to the VC group under TNF-α stimulation. This suggests that the Biofield Energy Treated test formulation has significant immunomodulatory activity. Scientific reports suggest that various immunological and inflammatory functions of chemokines play significant role in controlling the immune response during infections. Overall, the immunomodulatory effect might be the result of specific modulation of NF-κB, a transcription factor involved in the activation of many inflammatory mediator genes [45,46].
Worldwide scope of alternative medicine and its outcomes have been increased significantly. However, an important phytoconstituents along with minerals and vitamins are reported to have beneficial role against many diseases such as diabetes, indigestion, inflammation of intestine, osteomalacia, blood disorders, infertility, potent revitalizer, etc. [47]. Due to high safety profile with the wide therapeutic action of alternative medicines, the scope has been increased worldwide [48]. Besides, the individual constituents of the novel proprietary test formulation has been reported to have substantial immunomodulatory action, and Biofield Energy Healing Treatment significantly alters the action of cytokines. Overall, the Biofield Energy Healing Treatment on the test formulation can be a novel approach in supports of the use of Biofield Treated test formulation for various types of autoimmune disorders in colon cancer cell line (HT-29).
Conclusion
On the basis of current study findings, it is concluded that the novel proprietary test formulation showed significant antiinflammatory action on the tested cytokines (IL-6, IL-8, and IFN-γ) in colon cancer cell line (HT-29) after administration of the Biofield Energy Treated formulation. MTT assay in the Biofield Energy Treated colon cancer cell line (HT-29) suggest that the test formulation showed more than 78% cell viability and found as safe and non-toxic. In addition, the levels of cytokine, interleukin-6 (IL-6) was significantly reduced by 34.09% and 59.41% (p≤0.001) at 3 and 15 μM, respectively as compared to the vehicle control (VC) group. Moreover, IL-8 level was reported to be significantly suppressed in the Biofield Energy Treated test formulation by 57.09% and 42.88% at 0.1 and 3 μM, respectively as compared with the VC group. On the basis of experimental results of various tested cytokines and their expression, significant anti-inflammatory activity in colon cancer cells was reported in the new test formulation after treated with the Trivedi Effect®- Biofield Energy Healing. Biofield Energy Treated test formulation can be used as a Complementary and Alternative Medicine (CAM) to prevent the immune-mediated diseases such as Irritable Bowel Syndrome, Rheumatoid arthritis, Ulcerative colitis and Crohn’s disease, Stress, Asthma, and many more with safe therapeutic index. Besides, it can also be utilized in organ transplants (for example kidney transplants, liver transplants and heart transplants), various autoimmune disorders such as Lupus, Addison Disease, Celiac Disease (gluten-sensitive enteropathy), Dermatomyositis, Graves’ Disease, Hashimoto Thyroiditis, Multiple Sclerosis, Myasthenia Gravis, Pernicious Anemia, Aplastic Anemia, Sjogren Syndrome, Systemic Lupus Erythematosus, Diabetes, Alopecia Areata, Fibromyalgia, Vitiligo, Psoriasis, Scleroderma, Chronic Fatigue Syndrome and Vasculitis, Type 1 to improve the overall health and quality of life.
To Know more about Pharmacology & Clinical Research
Click here: https://juniperpublishers.com/index.php




No comments:
Post a Comment