Endocrinology and Thyroid Research - Juniper Publishers
Abstract
Background:TSH-secreting pituitary adenomas (TSH-omas) are very rare and an infrequent cause of thyrotoxicosis.
Case Report:A 7.9-yr-old boy was referred to
our Pediatric Endocrinology Unit due to a goiter. On admission, patient
was thyrotoxic with diffuse goiter. Laboratory evaluation suggested
inappropriate TSH secretion as the cause of hyperthyroidism: high serum
TSH in presence of elevated levels of TT4, TT3 and fT4, and TSH
unresponsive to TRH stimulation and to T3 suppression. Initially,
α-subunit (aSU) was in the upper limit of normalcy and pituitary MRI was
normal. One year after, patient was still hyperthyroid, despite regular
use of methimazole; TSH was 12.6 mU/mL, αSU was elevated and MRI
detected a pituitary 8 mm width adenoma, establishing the diagnosis of
TSH-oma. Peak GH (ng/dL) on ITT and TSH after TRH were 5.9 and 4.2,
respectively. Cortisol and prolactin (PRL) responded normally to ITT and
TRH tests. Transsphenoidal surgery was done and, postoperatively,
transient diabetes insipidus and adrenal insufficiency ensued. Two and
five months after surgery fT4, TT4 and TT3 were normal, albeit peak TSH
after TRH was 1.54. PRL and GH were unresponsive to adequate stimuli.
Fourteen months after surgery, TT4, TT3 and fT4 were low normal. He
presented with low IGF-1, low GH peak on dinanic tests and hypogonadism
and was treated with recombinant human growth hormone (rhGH) and
testostenone. At 16 yr-old, we reached final height, above target
height.
Conclusion: TSH-oma may be an etiology of
thyrotoxicosis in children. To our knowledge, this is one of the
youngest patients with TSH-oma yet reported.
Introduction
In most children with thyrotoxicosis the main cause
is Graves’ disease. Other causes include toxic adenoma, thyroiditis,
iodine-induced hyperthyroidism, McCune-Albright syndrome, syndrome of
resistance to thyroid hormone (RTH) and thytroid-stimulating hormone
(TSH) secreting pituitary adenoma (TSH-oma). TSH-oma comprises 0.5 to 3%
of all pituitary tumors. Patients present with signs and symptoms
related to thyroid hormone (TH) excess and/or to tumor size (headache,
visual field disturbances, cranial nerve palsies). The presence of
goiter is frequent [1,2]. Elevated TH levels in presence of
non-suppressed TSH should occur in TSH-omas, as well as in other
conditions such as early phase of destructive thyroiditis, irregular
replacement of l-thyroxine, assay interference of heterophilic
antibodies and RTH. The combination of high serum free TH,
inappropriately normal or elevated TSH, high serum α-subunit
(αSU) or increased αSU/TSH molar ratio and a pituitary tumor strongly
suggests the diagnosis of a TSH-oma.
Triiodothyronine (T3) suppression test is generally
reserved for patients with inconclusive results in above tests, because
genetic tests for detection of mutations in thyroid receptor (TR)α and
TRβ genes are expensive. Administering long-acting somatostatin analogs
has been proposed for distinguishing between thyrotropinomas and RTH,
since patients with thyrotropinomas would be likely to show a
significant reduction in free thyroxine (fT4) and T3 levels.
Approximately one third of patients with TSH-oma were misdiagnosed as
having primary hyperthyroidism and mistakenly treated with thyroidectomy
or radioiodine [2]. The majority of TSH-omas is monoclonal in origin,
like other types of pituitary adenomas. Pituitary-specific transcription
factor-1 (Pit-1) may play a role in adenomatous cell proliferation and
its overexpression was detected in growth
hormone- (GH), prolactin- (PRL) and TSH-secreting adenomas
more frequently than in normal pituitary. Reduced expression
of TR was demonstrated, and it could explain the abnormal
negative feedback of TH on TSH production by tumor cells [2,3].
TSH-omas are more fibrotic than other pituitary tumors
and it can worsen surgical outcome and somatostatin analog
treatment should be considered as the first-line treatment in
adults with macroinvasive TSH-omas [2,4,5]. Such an adenoma
is infrequent in adults and has rarely been report in children, we
describe an 8-yr-old boy with TSH-oma, and his follow-up until
final height. The patient and his mother assigned consentient
term.
Case Presentation
A 7.9-yr-old white pre-pubertal boy was referred to Pediatric
Endocrinology Unit due to goiter. His mother noticed he was more
irritable, and lost weight albeit an increased appetite. History
was negative for insomnia, headache or visual disturbance.
Physical examination disclosed a lean and hyperactive child with
stare opened eyes, warm and moist hands, with fine tremors.
Height was 138cm (1.78SDS; target height -0.96SDS), weight
27.2kg (0.40SDS), and BMI 14.28 (-1.14SDS). Pulse rate was
regular (108bpm) and blood pressure 100/60mmHg. Thyroid
was tender, diffusely enlarged (app.30g). Deep tendon reflexes
were exacerbated. Laboratory work-up revealed a bone age
(BA, Greulich & Pyle) of 9-yr, and the following thyroid function
profile (normal values in brackets) was found: TT3 181.9 (45-
137ng/dL), TT4 24 (6-12μg/dL), fT4 3.68 (0.71-1.85ng/dL), TSH
4.77 (0.49-4.67μU/mL); basal and peak TSH on TRH test 4.6 and
6.2, respectively; pre and post T3 suppression test RAIU (24h)
values were 42.1 and 30% respectively, while TSH did not change
significantly (4.15) whereas fT4 exhibited some reduction (2.64).
Anti-thyroid receptor (TRAb), anti-thyroglobulin (anti-TG) and
anti-thyroid peroxidase (anti-TPO) antibodies were negative.
Calcium and PTH levels were normal. αSU was 0.86 (≤0.8ng/mL),
αSU/TSH molar ratio 2 (<1) and magnetic resonance imaging
(MRI) of pituitary was normal.
Patient was managed with propranolol (1 mg/kg/day) and
methimazole (MTZ, 0.5 mg/kg/day) and thereafter, with MTZ
exclusively. Table 1 summarizes main preoperative clinical and
laboratory events. While on MTZ, T4 and T3 did not normalize,
TSH values ranged between 5.81 and 12.59 and goiter was
slightly enlarged. MTZ was withdrawn and thyroid and pituitary
functions were evaluated three weeks later. On combined insulin
hypoglycemia (ITT)/TRH tests, prolactin (PRL) and cortisol rose
properly, peak GH (ng/mL) was 5.9 and TSH was unresponsive
(Table 2). Basal LH and FSH were normal and IGF-I was 434 (30-
289 ng/mL). RAIU was elevated (75.8%) and rose paradoxically
(87%) after T3 suppression test. Sex hormone-binding globulin
(SHBG) was 233 (13-71 nmol/L), and T4-binding globulin (TBG)
was 16 (10-29 mg/dL). Repeated TRAb, anti-TG and anti-TPO
were negative. At this time αSU was high to 0.949 (αSU/TSH
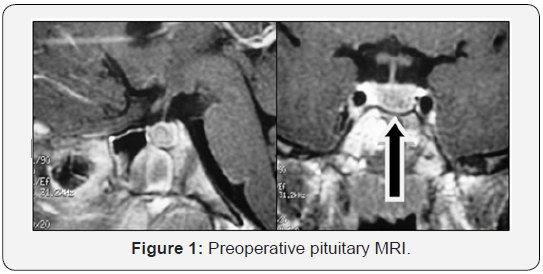
4.7) and pituitary MRI revealed the presence of an 8 mm width
microadenoma (Figure 1).
CA = Chronological age
vTranssphenoidal surgery (TSS) was performed and a
well-demarcated, fibrous and firm adenoma was excised.
The pathologic specimen showed adenoma cells that were
immunopositively only for TSH and chromogranin and negative
for LH, FSH, PRL, ACTH, and GH. Eighteen hours after surgery,
serum TSH and fT4 descended to 0.53 and 1.8, respectively and
goiter and thyrotoxicosis signs diminished as well. On the 3rd
day postoperatively, acute adrenal insufficiency and transient
diabetes ensued. Hydrocortisone and DDAVP were given and
maintained for 2 and 14 months, respectively. Two months
after TSS, ACTH was 12 (10-50 pg/mL) and IGF-1 64 (74-388
ng/mL). Peak GH and cortisol (μg/dL) on ITT were 0.2 and
26.9 respectively. PRL and TSH responses to TRH were blunted;
however, RAIU was normal (23.8%). One year after surgery,
BA was 11.5, TT3, TT4 and fT4 were in the low-normal range for
age, calorimetry was sub-normal and pituitary MRI showed no
evidence of tumor.
He had gained weight, but growth velocity was <1 cm/yr
despite adequate replacement dose of l-thyroxine (88 μg/day)
He was put on rhGH (0,033 mg/kg/day) and growth velocity
improved significantly (9.2 cm/yr). Three years after surgery, he
is still pre-pubertal and growing normally (on both l-thyroxine
and rGH). Last pituitary MRI was normal and aSU lower than
0.05 (aSU/TSH 0.61). His BA was 13.0 (chronological age 12.5)
and peak LH and FSH after GnRH were 1.1 and 1.4 mU/mL,
respectively. At that time, testosterone replacement was started
and after 9 months, he was pubertal. Five years after surgery,
rhGH was suspended, because he reached height above target
height. Six months later, testosterone replacement was stopped.
However, pubertal stage did not evolve and IGF-1 was 145 (226-
903 ng/mL), testosterone (250 mg/month) and rhGH (0.6 mg/
day) were re-started. At his last visit, at 16.3 yr.-old, height was
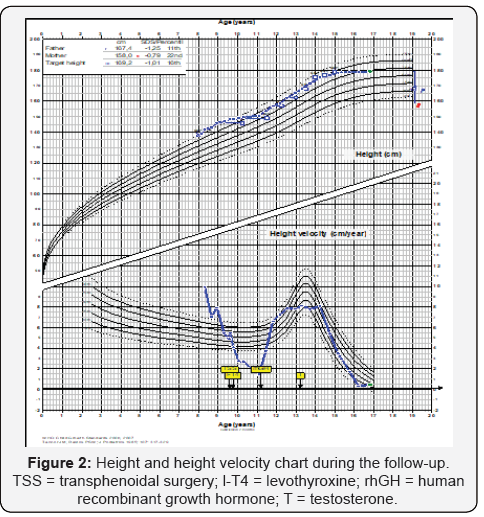
178.9 cm (0.59 SDS) and pituitary MRI was normal. Figure 2
shows his height and weight SDS during follow-up and table 3
summarizes main postoperative clinical and laboratory events.
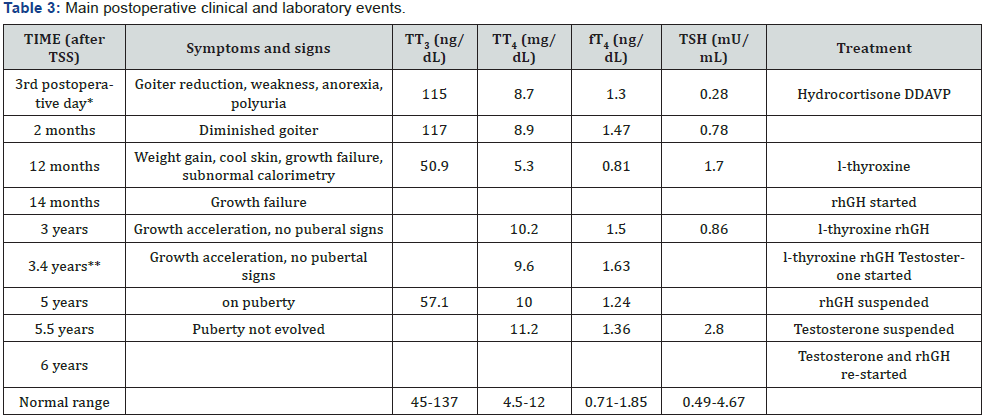
*Basal cortisol = 3.43 mg/dL (6-19); urine density =
1005; ** Total testosterone = 106 ng/dL (<100); peak LH and FSH
after GnRH = 1.1 and
1.4 mU/mL; CA = Chronological age; BA = bone age; rhGH = ecombinant
human growth hormone.
Discussion
Once inappropriate TSH secretion syndrome is identified,
specific investigation to differentiate a TSH-oma of RTH is
mandatory [2]. In our patient, TSH was not responsive to
TRH stimulation test and both aSU and aSU/TSH molar ratio
were high. In not previously treated subjects with RTH, the
TSH response to TRH is preserved, and aSU/TSH is normal.
Moreover, in RTH subjects a decreased secretion of TSH after
supraphysiological doses of TH is usually accompanied by a
reduction in RAIU [6], what was not observed in our patient.
These findings suggest that RTH was not likely. TSH-omas are
rare in adults and to our knowledge our patient is one of the
youngest children with hyperthyroidism due to TSH-oma ever
reported. Other 13 children or adolescents described were 8-yr.
or older (8 to 16yr) and had macroadenoma except a 13 yr-old
girl who had microadenoma and a 15 yr-old girl whose tumor
size was not decribed [7-19].
In this case, pituitary MRI suggested microadenoma, although
88% of TSH-omas are usually large and invasive [2,20]. Patient
underwent TSS because the primary goal of treatment of TSHomas
is, whenever possible, the complete removal of the tumor
[2]. TSH, TH and aSU levels reduced soon after surgery and one
week after, patient was euthyroid. TSS was successful in regard
the complete removal of the tumor, although in the follow-up
central hypothyroidism, and GH, PRL, LH and FSH deficiencies
succeeded. Panhypopituitarism and diabetes insipidus also have
been reported [14].
The first case of a patient with TSH-oma and normal aSU was
described in 1991 [21]. Valdes-Socin et al. observed normal aSU
in more than 60% of the cases. High aSU is often associated with
bad prognosis and was found more frequently in macro than in
microadenomas [4]. The high percentage of patients with normal
aSU could difficult differential diagnosis with RTH. Absence of
TSH response to TRH may be suggestive of presence of a TSHoma.
In difficult cases, genetic analysis looking for the presence of
a mutation in TRβ gene may easily help to discriminate between
the two disorders [2]. SHBG could also be a useful test yet its
level was almost invariably normal in patients with RTH but often
high in thyrotoxic patients with TSH-oma [2]. One challenging
situation is those patients with an invisible adenoma on MRI
and near-normal aSU, as initially occurred in our patient, whose
diagnosis was done one year after inappropriate treatment with
MTZ; possibly, that promoted tumor growth.
This case shows interesting aspects: the age of the patient
at diagnosis; the finding of a normal MRI in contraposition
to the elevated aSU/TSH molar ratio that was not adequately
interpreted; growth of the tumor during MTZ, blunted TSH
response to TRH in the post-operative phase in contradiction to
diminished calorimetry, low-normal values of fT4, TT4 and TT3,
and normal RAIU. We presented a comprehensive evaluation of a
patient with TSH-oma followed for more than 8yr, who attained a
final height, in accordance with the target height due to adequate
therapeutic management.
Learning points
i. Once inappropriate TSH secretion syndrome is
identified, specific investigation to differentiate a TSH-oma
of RTH is mandatory, even in children.
ii. Patients who have TSH-oma could be misdiagnosed
as having primary hyperthyroidism and, thus, mistakenly
treated with antithyroid drugs or thyroid ablation.
iii. TSH-oma may be a microadenoma and be present even
when aSU is near-normal or normal and it is a challenging
situation.
iv. After surgery, follow-up should be prolonged and
hormonal deficiencies should be diagnosed and treated.
To Know more about Endocrinology and Thyroid Research
Click here: https://juniperpublishers.com/jetr/index.php
Click here: https://juniperpublishers.com/jetr/index.php




I wasted a lot of money trying to find the right medication for my moms dementia all to no avail until Dr Erayo showed up and eradicated the stigma with the natural roots and herbs i ordered from him , my mom took it for 21days and she was cured from her dementia.
ReplyDeleteGod have use Dr Erayo herbal home to cure my mom, thank you so much Dr Erayo I am so happy. You can email Dr Erayo for help drerayoherbalhome@gmail.com
or whatsapp him on +2348151937428
website---- https://alternativeherbs.weebly.com
Youtube link---- https://www.youtube.com/channel/UCSp2m-_EHnCRQT4gYYTQWtg
FB page---- https://rb.gy/yuofn6