Journal of Head Neck & Spine Surgery
Abstract
The cellular therapy using Mesenchymal Stem Cells,
specially its two subtypes - Bone-marrow Mesenchymal Stem Cells and
Adipose Derived Stem Cells - can benefit by virtue of the possibility of
differentiating in specialized cells that secrete and suppress growing
factors and cytokines necessary in the lesion niche. When attracted by
the pro-inflammatory sinalization of the lesion, they act using the
paracrine signaling, decreasing the inflammation, increasing the
angiogenesis and the cell migration and proliferation. The development
in the researches regarding the association of the application of MSCs,
with reconstructive surgery practices, leads to effective future results
that can bring more benefits to the clinic practice of this field. This
paper has the objective of briefly reviewing the literature about the
usage of MSCs and its subtypes, the ADSCs, to improve the skin
cicatrization.
Keywords:
Mesenchymal stem cells; Adipose derived stem cells; Bone-Marrow
Mesenchymal Stem cells; Skin flaps; Skin grafts; Wound contraction;
Paracrine signaling; Pro-inflammatory Interleukins; Anti-inflammatory
Interleukins.
Abbreviations: MSC:
Mesenchymal Stem Cells; HLA-DR: Human Leukocyte Antigen; ADSC - Adipose
derived stem cells; BM-MSC: Bone-Marrow Mesenchymal Stem Cells; SDF1:
Stromal Cell-Derived Factor; PDGF: Platelet-Derived Growth Factor; IL:
interleukin; TNFα: Tumor Necrosis Factor-α; IFNγ: Interferon-γ; VEGF:
Vascular Endothelial Growth Factor; BFGF: Basic Fibroblast Growth
Factor; EGF: Epidermal Growth Factor; KGF: Keratinocyte Growth Factor;
HGF: Hepatocyte Growth Factor; PDGF: Platelet-Derived Growth Factor;
TGF-β: Transforming Growth Factor-β; FGF: Fibroblast Growth Factor;
α-SMA: Alpha-smooth muscle actin's; IRI: Ischemia-Reperfusion Injury
Introduction
Mesenchymal stem cells (MSCs) were first described by Friedenstein et al. [1,2]. MSCs are heterogeneous cell populations, called multipotent adult cells or somatic stem cells [3,4],
capable of differentiation in most cell types in order to maintain and
repair the organism. They are resident in different organs and tissues
such as adipose tissue [4,5], bone marrow, umbilical cord, amniotic membrane [2,4], kidneys, liver, spleen, lungs, pancreas, tendons, synovial membranes, placenta, amniotic fluid and dental pulp [4]. The International Society for Cellular Therapy proposed three minimal criteria for defining MSCs:
a) Plastic adherence;
b) Positive expression (greater than 95% of the cell
population) for CD105, CD73 and CD90 markers and negative, (maximum 2%
of the population) for CD45, CD34, CD14, CD11b, CD79a, CD19 and Human
leukocyte antigen (HLA-DR) surface molecules, and
MSCs have a great potential to the tissue repairment
and regeneration in reconstructive plastic surgery, preventing ischemic
lesions in skin flaps [7-14] and in skin grafts [15,16]. MSCs are capable of reducing severe atrophy, retraction, fibrosis and ulceration skin signals, caused by radiotherapy [17,18].
Combined with the lipoinjection in face and body defects and in
rejuvenating esthetic treatments, MSCs raise in 35% the survival rate
and the microvasculature from the injected fat [18].
Also, diabetic patients with ischemic wounds show greater survival
rates and lower number of amputations in affected limbs, when treated
with Adipose derived stem cells (ADSCs) [19]. The MSCs improved the skin healing at induced burns in rats [20,21]. In dermatology, the MSCs are being clinically used for inflammatory diseases [22-28], Graft-Versus-Host Disease [23,25], Chrown Disease [29], Systemic Sclerosis, Lupus or dermatomyositis [24,28,30].
When compared with the usage of Growing Factors, that
like any drug has a limited half-life, the cellular therapy using MSCs,
specially in its two subtypes - the Bone-Marrow Mesenchymal Stem Cells
(BM-MSCs) and ADSCs - can bring greater benefits because of: their
expansion capacity in cellular culture; stable phenotypic expression;
and possibility of differentiating in specialized cells, that secrete
and suppress cytokines and growing
factors in the injury [13,31-33].
However, long expansion periods of the cellular culture, to achieve
effective therapeutic doses, raise the possibility of infection from the
host. Because of that, the ADSCs still have to be heavily studied to
the application in the clinic routine [2].
This way, this study has as objective briefly review the literature
about the usage of MSCs and its subtypes, the ADSCs, to improve the skin
healing.
Discussion
The MSCs are capable to answer and adjust their functions, when exposed to cells or typical factors of the lesion environment [33,34]. They have an attraction capacity to the inflammation areas [33-35]
or tumors, because of the molecules CCL21 or HMGB1, known as homing. In
case of lacking of this signalization, they are attracted in order of
preference to the lungs, liver and spleen [35].
The MSCs show chemotaxis in vitro by the Stromal Cell-Derived Factor
(SDF1), Platelet-Derived Growth Factor (PDGF), Insulin-Like Growth
Factor-1, Interleukin-8 (1L-8) and Tumor Necrosis Factor-α (TNFα). In
murine models, the MSCs used in the systemic administration are capable
of getting into the damaged tissues [14,34,36].
The MSCs act, in many levels, in the three healing
phases: inflammatory, proliferative and remodeling phase. Current
studies indicate that the differentiation of the MSCs, that contributed
to the tissue regeneration, is limited by the small survival rate of
these cells in the damaged area. However, the paracrine signaling to the
cells from the injury is the main mechanism of the MSCs, reducing the
inflammation, stimulating the angiogenesis and inducing the cell
migration and proliferation [33,37].
Nevertheless, other authors suggest that the differentiation of the
MSCs in keratinocytes and endothelial cells have the same function as
the paracrine signaling to accelerate the neovascularization and the
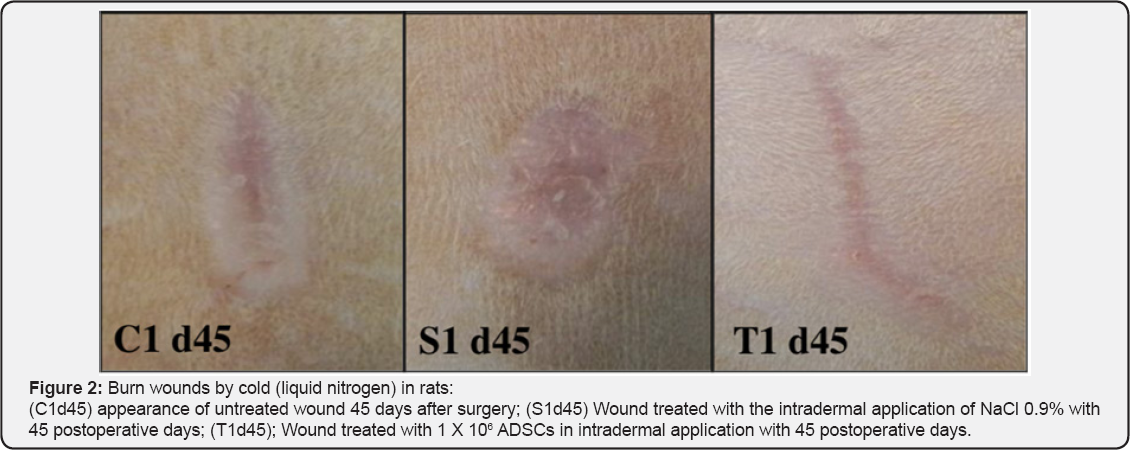
reepithelialization of wounds [32] (Figure 1 & 2).

Some studies suggest that immunosuppressive potential
is the same of both types of MSCs, ADSCs and BM-MSCs, in an
inflammatory environment. Their local administration inhibited the T
cells recruitment and proliferation [37-41] and decrease Interferon-γ (lFNγ),Tumor Necrosis Factor-α (TNFα), 1L-6 and IL-1β expression in the skin grafts [39-41].
In the inflammatory phase (one to three days), the expression of
anti-inflammatory cytokines 1L-4 and 1L-10 is increased while 1L-2 pro-
inflammatory cytokines, TNF-α and 1FN-γ are decreased, causing less
local inflammatory reaction [37,42].
MSCs can also regulate macrophage activation and convert phenotype
expression of M1 macrophages to M2 anti-inflammatory macrophages [39-41],
which accelerates the wound healing. Additionally, MSCs act over the
innate immunity to prevent the rejection of transplanted allografts,
inhibiting the complement system activation [39]. The decrease of TFN-α also helps the reepithelialization after the proliferation phase [43]. The antimicrobial action from the MSCs are also important to limit the infection occurrence [37].
With the utilization of cell therapy in the
proliferative phase (14 days) the production of important growth factors
is increased: Vascular Endothelial Growth Factor (VEGF), Basic
Fibroblast Growth Factor (bFGF) [32,37,42,43],
Epidermal Growth Factor (EGF), Keratinocyte Growth Factor (KGF),
Hepatocyte Growth Factor (HGF), Platelet-Derived Growth Factor (PDGF)
and Transforming Growth Factor-beta (TGF-β). Through their paracrine
action, the MSCs raise the migration and proliferation of keratinocytes,
endothelial and epithelial cells. The fibroblasts proliferation is also
enhanced, as well as the blood vessels' formation. This way, there is a
better growing of a more vigorous granulation tissue [33,37,42,43].
The angiogenesis is a very important factor to the wound's healing, and
the VEGF and the Fibroblast Growth Factor (FGF) are very important
angiogenic factors [32].
Through the paracrine signaling, from the MSCs in the
remodeling phase (21 days to one year), the fibroblasts produce
collagen fibers in greater quantity and density, generating an increase
in wound tensile strength [37,42,43].
At the same time, there is a smaller wound contraction and increased
matrix-metalloproteinases production, that control the collagen
deposition exacerbation, improving the aesthetic result and keeping the
skin's function [33,42,43].
MSCs also contribute to the scar's appropriate remodeling with the
increased secretion of VEGF and HGF, the adequate balance between TGF-β1
and TGF-β3 [37]
and the increase of bFGF and TGF-β which inhibit the Alpha-Smooth
Muscle Actin (α-SMA) expression, responsible for myofibroblast phenotype
[32].
Studies indicate that there is also the possibility
of using BM-MSCs and ADSCs to protect the damaged tissues caused by the
1schemia-Reperfusion 1njury (1R1) and to decrease the dysfunction of
organs undergoing ischemia. The ADSCs are able to protect axial flaps
from the 1R1 with results as satisfactory as the ones obtained in
procedures without ischemia, because of the improvement in the
angiogenic response and the blood perfusion elevation [10]. Many studies use the ADSCs to prevent ischemic injuries in skin flaps [7-14], as others [15,16] used them to improve the quality of skin grafts.
Conclusion
The MSCs - both BM-MSCs and ADSCs - are capable to
answer and module their functions, when exposed to cells or typical
factors of the lesion environment, attracted by the cell signaling to
the inflammation areas. The researches developed till the moment point
the paracrine signaling as the main mechanism from the MSCs, decreasing
the inflammation, benefiting the angiogenesis and inducing the cell
migration and proliferation in the damaged area. The progress on the
researches about the association of MSCs with reconstructive surgery
practices indicates good and productive results, which can benefit the
clinical practice in this field.
To Know More About Journal of Head Neck & Spine Surgery Please click on:




No comments:
Post a Comment