Peer Reviewed Chemistry Journals Juniper Publisher
Authored by Sureshbabu Jayachandra*
Abstract
The acid catalyzed cyclization of Benzyl substituted Octahydroisoquinoline results in the formation of a major product, N-Formyl Dextromethorphan and a minor product, one of the morphinan derivatives, which is formed via the mechanism of Super electrophiles. Excess Phosphoric acid acts as an electrophile and leads to the formation of a novel minor product but has a significant existence and can be isolated using Column Chromatography at the later stages.
Keywords: Morphinan derivatives; Alkaloids; Grewe cyclization; Super electrophiles
Introduction
Morphinan is the model chemical structure of a huge family of psychoactive drugs, containing opiate analgesics, antitussives and dissociative hallucinogens. It has a phenanthrene fundamental structure with A ring aromatic, the B and C rings being saturated, and an additional nitrogen-containing, six- membered, saturated ring, the D ring, attached to two carbons of the core, and with the nitrogen (Figure 1) [1]. If the D ring is “below” the core (dextro), the analgesic and euphoric properties are eliminated or are dramatically reduced, but the cough- suppressant property is retained, as in dextromethorphan [1].
Ofthe main naturally occurring opiates ofthe morphinan type- morphine, codeine and thebaine-thebaine has no therapeutic properties (it causes seizures in mammals), but it provides a low-cost feedstock for the industrial manufacture of at least four semi-synthetic opiates, counting hydrocodone, hydromorphone, oxycodone and oxymorphone, and, possibly more knowingly, the opioid antagonist naloxone [1]. The Benzyl substituted octahydroisoquinolines with a double bond common to the two fused rings served as a starting material in the synthesis of many morphinan derivatives. The synthesis of isoquinoline ring system is usually achieved by one of the traditional methods, such as Bischler- Napieralski reaction, Pictet- Spengler reaction or the Pomeranz- Fritsch reaction (Figure 2) [2].

So, Benzyl substituted octahydroisoquinolines (N-Formyl Octabase) is a raw material for the usual synthesis of Dextromethorphan Hydrobromide and one of the important intermediate is N-Formyl Dextromethorphan. The formation of this intermediate involves a well-known naming reaction, Grewe cyclisation [3]. The cyclisation has been performed using 85% phosphoric acid and toluene as a solvent (Scheme 1).
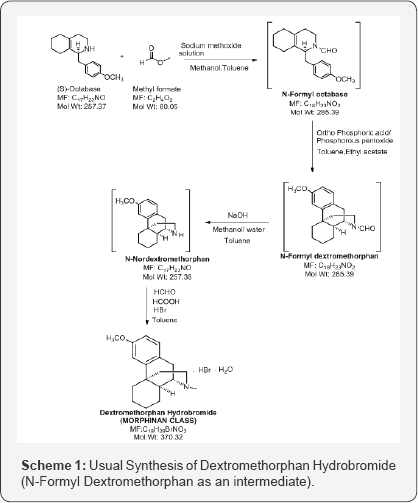
N-Formyl Dextromethorphan is further deformylated to N-Nor dextromethorphan and then methylated to form Dextromethorphan Hydro bromide salt (Scheme 1). In this manuscript, only the preparation of N-Formyl Dextromethorphan from N-Formyl octa base is projected and the formation of a significant Morphinan derivative.
Results and Discussion
The reaction of N-Formyl Octa base with phosphoric acid present in excess act as a catalyst, to form cyclized derivative, N-Formyl Dextromethorphan, using a well- known Grewe cyclization. Grewe cyclization is a synthetic methodology involving the formation of superelectrophiles. Superelectrophiles are multiply charged cationic species (dicationic, tricationic etc.) which are usually characterized by their reactions with weak nucleophiles like arenes, alkenes or alkanes. This mechanism involves chargely densed species formed because of super acidic media or excess acid catalyzed. Due to this, it leads to the double protonation of the molecule resulting in the reactive dicationic intermediate [4].
The two basic categories of superelectrophiles are:
a) Gitionic (close) Superelectrophiles - where charge centres are separated by no more than one carbon atom or heteroatom. i.e. 1,2-dicationic or 1,3- dicationic.
b) Distonic (distant) Superelectrophiles- where charge centres are seperated by 2 or more carbon atoms i.e. 1,4- dicationic or 1,5- dicationic.
Amongst the reactions involving superelectrophiles, a significant number involve the migration of carbon atoms, as in the case of our lab molecule that undergo ring expansion. N-Formyl Octa base when treated with excess amount of acid, so, the olefinic group in isoquinoline ring being a weak nucleophile, gets protonated along with the protonation of nitrogen atom. Here, protonation of nitrogen atom is the key element explaining the mechanism and the success of this reaction. Thus, the resulting molecule is 1,4- dication. The notable feature in this intermediate is that, tertiary carbocation is formed, therefore, a stable intermediate. Due to charge electrostatic repulsive effects, protonation at the double bond is regioselective forming 1,4-dication opposing the formation of 1,3-dication generating Distonic super electrophiles (more charge separation) (Scheme 2) [5].
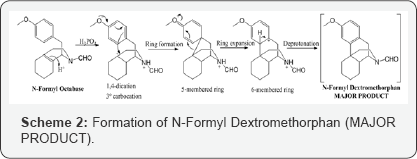
Now, again the electron rich arene will attack the carbocation to form 5-membered ring which is expanded to form fused 6 membered rings. This step can be explained that superelectrophiles undergo structural rearrangements that lead to favorable deprotonation step and this gives molecule with least or no positive charges. Thus, we achieve our major product and is formed because of most favorable mechanism. The mechanism adopted by superelectrophiles concluded that deprotonation- reprotonation is one of the most common path by which charge migrates in superelectrophiles. Rapid deprotonation in the strongest superacids is because of these reactive intermediates exhibiting extreme level of carbon acidity. Phosphoric acid (85%) in toluene is distilled using Dean- Stark apparatus to remove excess water azeotropically. The obtained phosphoric acid is approximately 97% and then phosphorus pentoxide is added under nitrogen atmosphere to obtain 100% phosphoric acid. Further, a solution of N-Formyl Octa base in toluene is added dropwise and the reaction is heated and stirred at 65-70oC for 12 hours to obtain the desired product (N-Formyl Dextromethorphan). The completion of reaction is analyzed by HPLC monitoring.
This N-Formyl Dextromethorphan is further insitu reduced to N-Nor Dextromethorphan using sodium hydroxide and methanol/ water mixture and then reacted with formic acid, formaldehyde and hydro bromic acid to form Dextromethorphan Hydro bromide (Scheme 1). During isolation of Dextromethorphan hydro bromide and LC-MS data, showed the formation of an unknown compound with same mass as Dextromethorphan but at different Retention time. The structure of this compound is analyzed and characterized using techniques like HPLC, 1H NMR, 13C NMR and DEPT (Figure 3).
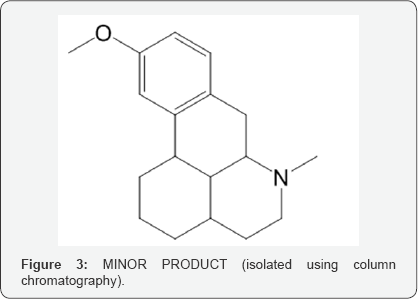
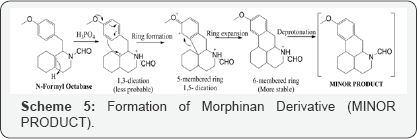
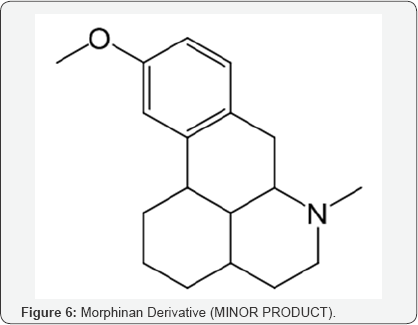
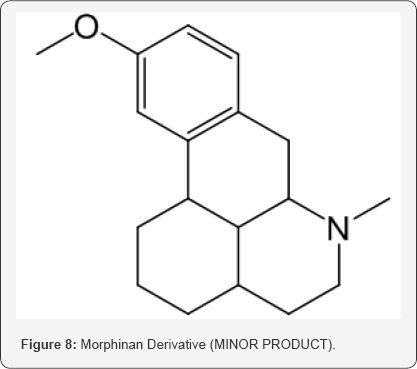
Considering all the insitu reaction stages and the possible by products, it is worthwhile to propose the formation of this compound from its precursor, originated in the step of Grewe cyclisation of N-Formyl Octabase although in minor quantity (Morphinan derivative) (Scheme 3).
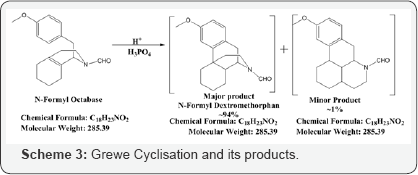
As all the 4 stages are carried forward in situ, and isolation is done only at the Dextromethorphan Hydro Bromide stage, the preparation of this minor product from its N-Formyl precursor takes place in the same way as N-Formyl Dextromethorphan to Dextromethorphan Hydrobromide. It has been in situ reduced to N-Nor using sodium hydroxide and methanol, and on treatment with with formaldehyde, formic acid gives Dextromethorphan Hydrobromide (~95%) along with this minor morphinan derivative (~1-2%) as per monographs of European Pharmacopoeia (EP) [6], United States Pharmacopoeia (USP) [7] and International Pharmacopoeia (IP) [8]. This minor product is isolated (at this stage) by column chromatography (MDC: MeOH :: 9:1) followed by Preparative HPLC (Scheme 4).

The formation of this intermediate can be explained with the possibility of formation of 1,3-dication over 1,4-dication (which is less probable). Another difference lies in the arene attack which attacks the carbocation from the comparitively less electron rich position and only reason to justify is the resulting separation of charges and formation of 1,5-dication resulting in 5-membered product which is expanded to form fused 6 membered rings with carbon migration, giving us the minor product (Scheme 5).
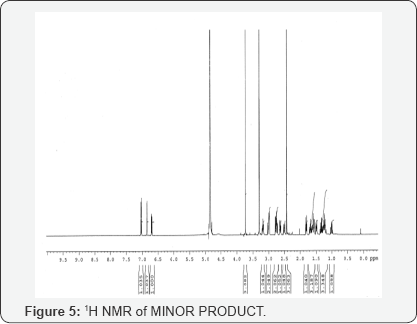
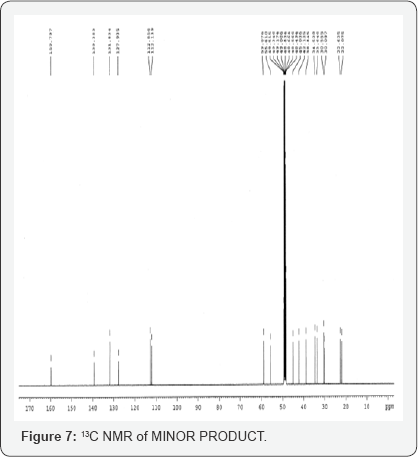
This isolated minor product is analyzed and characterized using Mass spectrometry, HPLC and NMR (1H and 13C NMR). DEPT- analysis showed the presence of 6 -CH2 groups instead of 7 -CH2 groups in the major product. All the characterization and analysis confirmed the formation and structure of the isolated minor product (Figures 4-9).

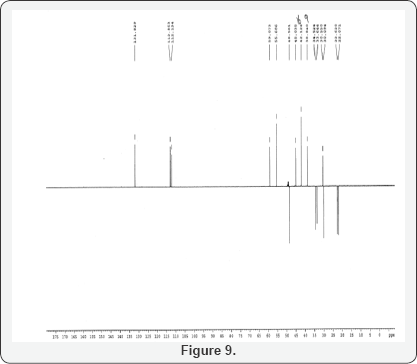
Dept of MINOR Product
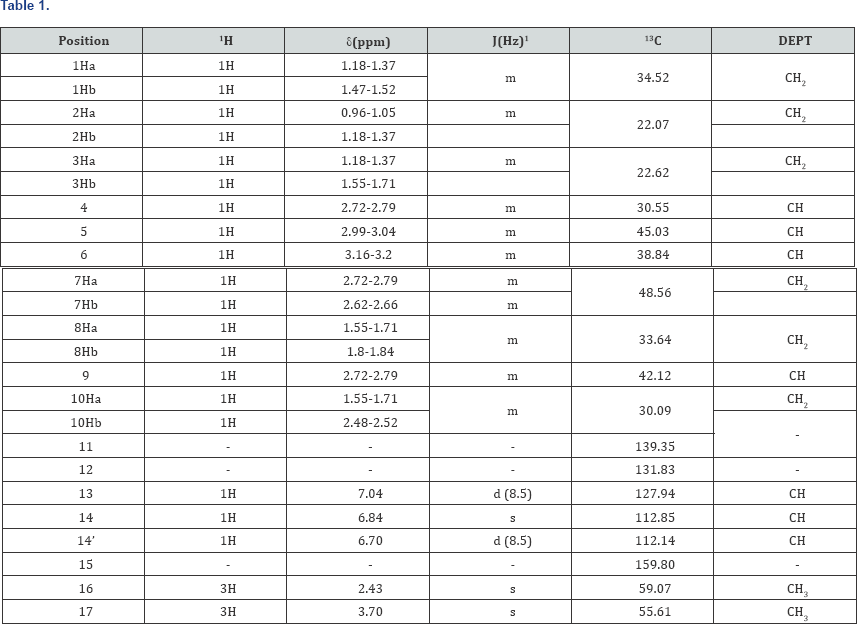
s- singlet, d- doublet, m-multiplet, br-broad, 1H-1H coupling constants.

Experimental Section
Preparation of N-Formyl Dextromethorphan (MAJOR) and Morphinan Derivative (MINOR)
In a flask, charge Ortho phosphoric acid (~85.0 % w/w). Charge Toluene and Raise the temperature of the reaction mass. Reflux and maintain over Dean stark apparatus to remove water azeotropically. Cool the reaction mass under nitrogen atmosphere and Charge Phosphorus pentoxide under nitrogen atmosphere. Slowly add N-formyl octa base organic layer and Raise the temperature of the reaction mass under nitrogen atmosphere. Stir and maintain the reaction mass at 65-70oC under nitrogen atmosphere till reaction complies by reaction monitoring by HPLC after 10-12 hrs maintenance. Cool the reaction mass under nitrogen atmosphere. Concentrate the reaction mass under vacuum to remove toluene. To the concentrated mass charge ethyl acetate under nitrogen atmosphere and stir. In another flask, charge water, Cool. Charge ethyl acetate reaction mixture reaction mass in to chilled water. Stir, settle and separate the layers. Wash the organic layer with water again and then a wash of 7% sodium bicarbonate solution is given. Concentrate the organic layer u/v till almost no solvent distills.
Preparation and Isolation of MINOR Product
N-Formyl Minor product obtained in Grewe cyclisation, is treated with Sodium hydroxide in the mixture of methanol and water for 12 hours at 65-70oC to give N-Nor which is further treated with formaldehyde and formic acid in the presence of toluene for two hours at 60-65oC to give N-Methylated minor product which is further purified and isolated using Column chromatography and Preparative HPLC.
Acknowledgements
Our group would like to thank the Department of Scientific and Industrial Research India, Dr. Hari Babu (COO Mylan), Sanjeev Sethi (Chief Scientific Office Mylan Inc ) ; Dr Abhijit Deshmukh (Head of Global OSD Scientific Affairs); Dr Yasir Rawjee {Head - Global API (Active Pharmaceutical Ingredients)}, Dr Sureshbabu Jayachandra (Head of Chemical Research) Mr Manoj Pananchukunnath (Head of Global Injectables Scientific Affairs, Product Development) Dr. Suryanarayana Mulukutla (Head Analytical Dept MLL API R & D) as well as analytical development team of Mylan Laboratories Limited for their encouragement and support. We would also like to thank Dr Narahari Ambati (AGC- India IP) & his Intellectual property team for their support.
To
Know More About Peer Reviewed Chemistry Journals Please Click on:
https://juniperpublishers.com/omcij/index.php
To Know More About Open Access Journals Publishers Please
Click on: Juniper Publishers




No comments:
Post a Comment