Peer Reviewed Chemistry Journals Juniper Publishers
Abstract
Classical chelating agents (especially aminopolycarboxylates, APCs and phosphonates) are till date the commonly used in industrial and home processes. This is due to their ability to strongly bind metals, and perhaps their being available in the market over a long time now. Abundant evidences proved that they are not environmentally benign. This has spurred the quest of industrialists and academia, as prompted by environmental policies, towards low toxicological profile and environmentally friendly chelating agents. The desire has resulted into annual rise in formulations and proposals of Greener alternative chelators such as glutamic diacetic acid (L-GLDA), ethylenediamine disuccinic acid [S, S]- EDDS, polyaspartic acid, citrate, gluconic acid, amino acids, lipophilic β-diketone (14,16)-hentriacontanedione, plant extracts etc. to be used in place of the classical chelating agents. For reasons of environmental compatibility, low toxic profile and sustainability, these Greener alternative chelants are better employed for industrial uses (such as metals extraction and recovery etc.) and home applications.
Keywords: Greener Chelators; Metals Recovery; Stability Constant
Introduction
Metals are extensively used by industries in various applications such as electronics, materials, catalysts, chemicals, modern low-carbon energy technologies [1] (nuclear, solar, wind, bioenergy, carbon capture and storage (CCS)) and electricity grids [1,2]. Greater pressure has been placed on metal utilisation because of population growth coupled with a higher standard of living. Furthermore, industrialisation has led to the increasing demand for critical metals, as many of these are required in modern technologies. This is causing concern over the supply of critical metals for future generations. Therefore, according to Hunt et al. [3] the sustainable use of metals is vital so that both the current and future generations have access to them without hitches. Industries or nations classify metals as critical depending on the purpose and need of assessment [4]. Some metals have been identified as critical metals because of their significance [3]. However, elements with significant supply restriction issues (geopolitical issues, conflicts, international monopolies and mining as a by-product of other elements) and those which would have a dramatic impact on business or economy if limited are considered critical [5]. The top 14 metals like tellurium, indium, tin, hafnium, silver, dysprosium, gallium, neodymium, cadmium, nickel, molybdenum, vanadium, niobium and selenium are critical and commonly needed in these emergent low carbon energy technologies [1,6].
Furthermore, industries generate a variety of wastes which contain heavy metals [7]. Electroplating and mining companies generate large amounts of mercury, lead, cadmium, silver, copper, and zinc ions [8,9]. More so, papers, metals, electrical and electronic equipment wastes contain precious metals like Ag, Au [10]. And some of these metals are regarded as technology metals [11,12]. Unfortunately, the reserves of high-grade ores of these metals are depleting [7]. Therefore, there is a need to recycle and recover these metals from the environment. Moreover, some heavy metals can be hazardous even at low concentrations [9,13]. According to Nagajyoti et al. [14] heavy metals such as Cd, Cu, Pb, Cr and Hg are major environmental pollutants, particularly in areas with high anthropogenic activities. Thus, when these metals are in bioavailable forms and at excessive levels, they have the potential to become toxic to plants and consequently the environment [14]. In addition, there are usually trace amounts of iron, copper, manganese, calcium, and other metals found naturally in many raw materials [15,16]. These metal ions are normally found in processing water as well, and may infiltrate processing [15]. Moreso, presence of metal ions in a process or product can bring about scaling, chemical degradation, discoloration, precipitation, emulsion instability, rancidity, and reduce; quality, consumer appeal, shelf-life and ultimate value [15,17,18].
Fortunately, chelating agents have been used to eradicate these problems by binding metal ions via N, O, S atoms as the case may be [19]. When metal ions are bonded to chelant, the metal becomes blocked from undesired interaction [20]. Hence, Chelating agents find use in paper pulp bleaching [21,22], detergents and cleaning [23], water treatment and food industries [24,25]. Chelants have also been used for the extraction of metals [22,26-33]. Other applications where chelating agents are used include fertilisers [22,34-38], photography [39] and pharmaceuticals [40,41]. They are also applied in nuclear industry, soil remediation [42,43] and textile treatment [44]. Additionally, chelators are used in many products to prevent; chemical degradation, discoloration, precipitation, emulsion instability and rancidity; thus increasing consumer appeal, shelf-life, and ultimate value [17]. Chelants are also used for heavy metal detoxification [45], treatment of antitumor [46] and in radioimmuno-diagnostics [47,48]. They are potent agents for solubilising heavy metals from polluted soils [44,49–51] and as root canal lubricants [52]. Sometimes chelating agents are used as precursors of catalysts [53,54]. Chelants are also used to prevent scale [22,55]. They can also enhance the growth of plants by removing toxic metals from the soil [56,57]. Because of their wide needs, the overall chelating agents growth was 4.0% annually during 2009-2014 [58] and the trend is likely to increase..
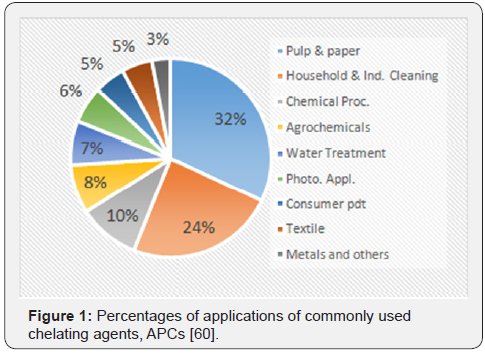
Incidentally, classical chelating agents such as aminopolycarboxylates, aminophosphonates and phosphates are used to chelate metals with substantial stability [8]. Phosphonates are extensively used as scale inhibitors [20,59]; and as ingredients in detergents for cleaning processes due to their ability to effectively bind Ca(II) [20]. Traditional phosphonate chelating agents include: diethylenetriaminepentakis(methylenephosphonic acid), DTPMP; 1,2 –diaminoethanetetrakis (methylenephosphonic acid), EDTMP; 1-hydroxy ethane (1,1-diylbis-phosphonic acid), HEDP; phosphonobutanetricarboxylic acid, PBTC; nitrilotris (methylenephosphonic acid), NTMP; N-phosphonomethylgycine, PMG. While the aminopolycarboxylates (APCs) chelants mostly used as chelators include ethylenediamine tetraacetic acid, EDTA; nitrilotriacetic acid, NTA; β-alanine diacetic acid, ADA; diethylenetriaminepentaacetic acid, DTPA; ethylenediaminedi(ohydroxyphenylacetic acid, EDDHA; N- (hydroxylethyl) – EDTA, HEDTA [40]. APCs and phosphonates are among the most widely used chelating agents in the world, accounting for 37.8% of consumption in 2009 [8,58]. Figure 1 below presents the percentage distribution of the most consumed chelating agents across different sectors [60].
Aminopolycarboxylates (APCs) chelators (like EDTA and NTA) and phosphonates have strong chelation effects for metals [20,61]. Unfortunately, most of these compounds are not readily biodegradable [20,59,62]. The infiltration of these chelants into the environment could cause dissolution of heavy metals from the sediments and soils, thereby mobilizing them [24,40,49,63] thus leading to increased levels of metals [22], except phosphonates that do not mobilise toxic metals [40,59]. These strong chelants persist in the environment due to their high solubility in water and low biodegradability (except NTA) [22]. It has been stated that 800 μg/L of EDTA has been found in some U.S. industrial and municipal wastewater treatment plants and up to 12 mg/L in European bodies of water [20]. EDTA is now among the EU priority list of substances for risk assessment [16]. According to Sillanpaa [64], ethylenediamine tetraacetic acid (EDTA) contains 10% nitrogen which could harm aquatic organisms. Furthermore, the majority of the traditional chelating agents (APCs and phosphonates) are petroleum derived [65,66]. Therefore, the consumption of traditional APCs chelators is declining (–6% annually), because of the persisting concerns over their toxicity and negative environmental impact [58]. Another concern is that most of these common chelants are produced from toxic substances like cyanide [20,67].
In addition, the EU is regulating the use of phosphates in consumer laundry detergents and consumer dishwasher detergents in order to reduce the eutrophication risks and costs of phosphate removal by wastewater treatment plants [68-71]. Their persistence in the environment is because of their low biodegradability and high water solubility [67,72]. In addition, studies have shown that there is a decline in the high quality phosphorus rock reserves used to produce phosphate chelants which could lead to higher costs associated with obtaining phosphates and phosphonate products. The continuous dependence on phosphates and phosphonate chelators will further accelerate the decline of finite high quality phosphate rocks [73]. Furthermore, phosphates are essential components in fertilisers (used for food production) and therefore the utilisation of phosphates as chelators is in direct competition with the food industry. Therefore, it is essential to look for Greener alternative chelating agents in order to reduce the reliance on these traditional chelants. Hence, this paper gleans for the Greener alternative chelators and their applications, especially in metals recovery.
Materials and Methods
Aminopolycarboxylic acids chelators are the most widely consumed chelating agents; however, the percentage of the Greener alternative chelators in this category continues to grow [24]. In 2013, these Greener alternative chelants represented approximately 15% of the total aminopolycarboxylic acids demand. This is expected to rise to around 21% by 2018, replacing in particular the EDTA (ethylenediaminetetraacetic acid), NTA (nitrilotriacetic acid) and aminophosphonic acids used in cleaning applications [20,24,58]. This is because of issues like non-biodegradability, toxicity, and mobilization of toxic metals by these traditional chelants [24] as earlier mentioned. In addition, more than 90% of organic chemicals are derived from fossil fuel refineries [74,75] which is not sustainable. The continuous depletion of petroleum resources coupled with a shift to Greener products by consumers means that it is vital to look for alternative Greener chelating agents. Therefore, in order to replace traditional chelants, the alternative chelating agents must have a strong ability to form complexes [16,76], as well as possess low nitrogen content so as to reduce the loading of nitrogen [16]. In addition, they should be readily or at least inherently biodegradable [16,76]. These alternative chelants are well favored by environmental protection policies [62,77]. Examples of some Greener alternative chelating agents include ethylenediamine disuccinic acid ([S, S]-EDDS), polyaspartic acid (PASA), methylglycinediacetic acid (MGDA) [24,25], glutamic diacetic acid (L-GLDA), citrate, gluconic acid, amino acids, plant extracts etc. Asemave [78] and Asemave et al. [79] reported the use of lipophilic β-diketone, 14,16-hentriacontanedione as Greener alternative chelator for metals recovery. These have been proposed to replace the classical EDTA and diethylenetriaminepentaacetic acid (DTPA) chelators in various applications [16,20,80,81]. According to Hyvönen [16], alternative chelants have a lower chelating ability when compared to the traditional chelators, notwithstanding, this will make them less toxic.
Glutamic Acid Diacetic Acid (L-GLDA)
L-glutamic acid diacetic acid is 86% bioderived from foodapproved natural amino acid salt (monosodium L-glutamate or MSG) [66,82]. It’s in turn obtained by fermenting sugar, molasses, corn or rice (renewable feedstock) [66], and is marketed as Dissolvine GL-38 [24]. According to Dixon [24], L-GLDA is produced by a waste-free process and from renewable feedstock, which is in accordance with the 4th principle of green chemistry [24]. Ammonia is generated as a by-product which is collected and re-used in industries. It is a strong chelating agent that is safe, readily biodegradable [61,83] and is considered to be an adequate alternative to phosphates, NTA and EDTA, especially in cleaning applications [20,61,84]. It is readily soluble in water at different pH values, which increases its performance rate [20]. L-GLDA is stable over a different temperature than other APCs. L-GLDA, citrate and carbonate are incorporated in detergent formulations [85]. Aqueous solutions containing L-GLDA can be use as oil field chemicals to dissolve calcium carbonate scale and other subterranean carbonate formations to increase permeability and enhance the withdrawal of oil or gas [86].
Polyaspartic Acid
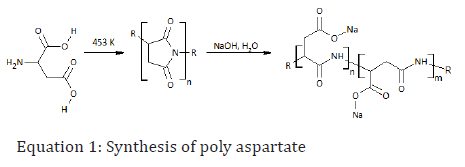
There are different ways to obtain PASA [87], but the typical method for obtaining it is by heating aspartic acid to 453 K resulting in poly (succinimide) with elimination of water. The sodium hydroxide in the system then reacts with the polymer to partially cleave off the amide bonds, in which the (α and β) bonds are hydrolyzed resulting in a sodium poly (aspartate) copolymer with 30% α-linkages and 70% β-linkages (see Equation 1) [87]. Polyaspartic acid production (PASA) is cost-effective; hence it is available on a large scale. L-aspartic acid derived from plant sugars [88] could be used for the sustainable production of PASA. Poly aspartate is used as a biodegradable anti-scaling agent, corrosion inhibitor and as a metal chelator [87]. Lingua et al. [89] described PASA as a green chelant used in agriculture to supply minerals to crop so as to improve the crop yield.
Ethylenediamine Dissunic Acid ([S, S]-EDDS)
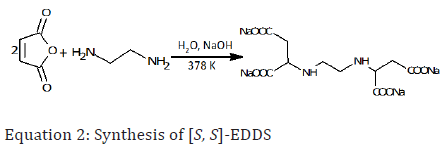
Ethylenediamine disuccinic acid, [S, S]-EDDS is a naturally occurring compound and was first isolated from culture filtrate of the actinomycete, Amycolatopsis orientalis. The biosynthesis of [S, S]-EDDS is from L-aspartate and serine [83,90] or from oxaloacetate and 2,3-diaminopropionic acid [35]. [S, S]-EDDS is also synthesized by the nucleophilic addition of ethylenediamine with sodium maleate affording stereoisomers of ethylenediamine-N,N’-disuccinic acid [90,91]. Alternatively, [S, S]-EDDS is produced from the reaction of maleic anhydride and ethylenediamine to yield a mixture of the 3- isomer of EDDS. The reaction of aspartic acid with 1,2-dibromoethane results in the formation of two isomers ([R, R]-EDDS and [S, S]-EDDS, depending on the isomer of aspartic acid used. Since aspartic acid can be derived from plant sugars it could also enhance the sustainable production of [S, S]-EDDS. It is also produced by fermentation of A. orientalis [35]. [S, S]-EDDS is the structural isomer of EDTA, however it is readily biodegradable than EDTA [61]. Equation 2 describes the synthesis of this compound.
According to Dixon [24], [S, S]-EDDS production is in conformity with the 3rd principle of green chemistry; i.e. designing less hazardous chemical synthesis. It is one of the most promising biodegradable chelating agents [39,49] and has a low nitrogen content [16] making it less toxic [92]. Furthermore, [S, S]-EDDS has zero NTA, formaldehyde or cyanide (toxic chemicals) unlike common traditional APCs chelants [83]. [S, S]- EDDS is effective in chelating several metals from soil [93-95]. Furthermore, it is capable of binding transition metal ions in place of Mg(II) and Ca(II) [24,83]. According to work by Yang et al. [96] [S, S]-EDDS at pH 5.5 is more suitable for Cu(II), Zn(II) and Pb(II) extraction. Ullmann et al. [97] modified [S, S]-EDDS by attaching a lipophilic hydrocarbon chain to its nitrogen atoms in order to make a hydrophobic chelating agent. Such lipophilic chelants are especially good as metals extractants.
In addition to these chelating agents above, in 1998, another greener alternative chelator, sodium iminodisuccinate was introduced [20]. Its production is based on the reaction of maleic anhydride with ammonia and sodium hydroxide [20,98] (see Equation 3). It is readily biodegradable [43,99] and environmentally benign chelator, it is effective in chelating Ca(II), Fe(III), Cu(II). And is used in; cleaning, water softening, photography, agriculture. Thus eliminating the problem of environmental persistence common conventional chelating agents [20]. Moreso, we have methylglycine diacetic acid (MGDA) as greener chelant. In fact, methylglycinediacetic acid (MGDA), L-GLDA and ethylenediamine disuccinic acid [S, S]-EDDS can be used in scale inhibition [100]. Both [S, S]-EDDS and MGDA have demonstrated to be efficient chelating agents with a mobilizing capacity that is comparable with EDTA [43]. MGDA is also considered as a possible replacement for EDTA and DTPA [40]. It is one compound which has been considered as a good substitute for EDTA and DPTA like [S, S]-EDDS [40]. Another biodegradable chelating agent, tetrasodium 3-Hydroxy-2,2’-Iminodisuccinate (HIDS) has also been reported to have high chelating capability [101], which is effective in removing heavy metal ions such as Fe(III), Cu(II), Ca(II) and Mg(II) over wide range of pH. It’s thermally stable, solubility in concentrated alkaline solutions, and is and environmental harmonious chelating agent [101]. HIDs is being found applicable in cleaning processes, textile processing, bleach stabilization, photography, paper and pulp processing, scale removal and prevention, metal treatment working, water treatment, Agriculture [101,102].

Citrates
These are salts of citric acid (2-hydroxy-1, 2, 3-propane tricarboxylic acid). Citric acid is known to be produced by fermentation (using fungi and yeasts) [103], synthesis and extraction from citrus fruits [103,104]. Vegetable wastes of potato, brinjal, cabbage wastes also have been found to as potential sources of citric acid [103]. Citrate fruits are used in the treatment of renal calculi [105]. Citric acid is an excellent chelating agent which is used to remove lime scale from boilers and evaporators [87]. They are used in some cases in place of classical chelating agents. For instance, a 24 h washing of the contaminated soil with 0.5 M citric acid reduces the levels of Cd(II), Cu(II), Zn(II) and Pb(II) from 0.01, 0.04, and 0.42, 41.52 mg g-1 to 0, 0.02, 0.18, and 5.21 mg g-1 respectively [106]. In another development, the ability of citric acid as chelating agents to the removal of lead from contaminated soil was examined both in the soil washing [107]. In the soil washing, the removal efficiency of lead with citric acid was less as [S, S]-EDDS in the pH range from 7-10 [107]. Although citrate is less efficient in terms of coordinating metal ions as compare to some conventional chelants, its activity towards removal of Pb(II) in acid soil is better for its low cost and less harm to crops [42]. It is also used for removing Ca(II) ions [87]. Citric acid is green chelator for removal of heavy metals from contaminated sludge with higher extraction efficiency at mildly acidic pH of about 2.30 [107- 109]. Citric acid was found to highly efficient for the recovery of Cr(III), Zn(II) and Mn(II) from a printed circuit boards (PCBs) [110]. Again mobilization of Pb(II), Zn(II) and Cu(II) from harbor sediments using citric acid as chelating agents has been previously reported [95]. Extraction efficiencies of citric acid for Cr(III), Cu(II), Ni(II), Pb(II) and Zn(II) is significant to lower the heavy metal content in sludge below the legal standards [111].
Gluconates
Gluconic acid (C6H12O7) is found naturally in fruit, honey, kombucha tea, and wine [87]. Gluconic acid is a weak organic acid obtained from glucose by a simple oxidation reaction. The oxidation is done by the enzyme glucose oxidase (fungi) and glucose dehydrogenase (bacteria such as Gluconobacter) [112]. But the microbial production of gluconic acid is the preferred method where the most studied and widely applied fermentation process involves the fungus Aspergillus niger [112]. Gluconic acid has two bonding sites: the ionic acid oxygen (-COO-) and the oxygen on the hydroxyl group (-OH) which can bond with the metal ion [113]. Gluconic acid and its derivatives (such as the sodium gluconates) have wide applications in food and pharmaceutical industries because of their chelating ability [112,113]. Aqueous solutions of the natural chelating agents D-gluconic acid and D-glucaric acid (D[+]-saccharic acid) were used to remove heavy metal ions (Cd(II), Cr(III), Cu(II), Ni(II), Pb(II), Zn(II)) from a soil polluted by long-term application of sewage sludge [114]. They found that, between the pH 12.0 and 13.0, Pb(II) and Cu(II) were selectively extracted [114].
Gallic Acid
Bioconversion studies with Aspergillus niger and Rhizopus oryzae showed that raw substrates like myrobalan fruits can be used as potential substrates instead of extracted tannins for gallic acid production [115]. It was found that Aspergillus niger is better gallic acid producing strain [115]. Gallic and citric acids were reported to induce removal of Cd(II), Zn(II), Cu(II) and Ni(II) from soil without increasing the leaching risk [63]. Net removal of these metals by these acids can be as much as other classical chelators. A major reason for this is the lower phytotoxicity of gallic and citric acids [63]. Other bioderived molecules like cyclodextrins (CDs) have also been identified as molecular chelating agents [116]. Cyclodextrins possess a cage-like supramolecular structure like cryptands, calixarenes, cyclophaneS, Spherands and crown ethers [116]. It can either be in alpha, beta, or gamma form cyclodextrins [80]. Therefore CDs complexes are widely used in many industrial products, technologies and analytical methods [116]. Other applications includes; drug carrier, food and flavors, cosmetics, packing, textileS, Separation processes, environment protection, fermentation and catalysis because of negligible cytotoxic effects of CDs [116]. Also, phytochelatins are oligomers of glutathione, produced by the enzyme phytochelatin synthase. They are found in plants, fungi, nematodes and all groups of algae including cyanobacteria [87]. Phytochelatin are used for heavy metal detoxification [87]. Another natural chelator, phytic acid is an organic acid found in rice bran [117]. It is used as an acidulant for pH adjustment. Phytic acid binds to metals strongly because of strong chelating effect [117]. Moreover, phytic acid shows antioxidant action and prevention of color degradation [117]. The most outstanding feature of phytic acid is its strong metal chelate function, allowing metal ions such as iron (Fe) which often adversely affect the production or storage of food in various forms to be removed or deactivated [117]. Moreso, pectin (found in foods like, apples, bananas, grapes, okra, beets, carrots and all citrus fruits) is useful in removing of heavy metals from the body [118].
Chitosan is a useful polymeric material produced from the shells of crustaceans [119]; it’s a partially deacetylated polymer of acetylglucosamine [119]. Chitosan is a common biodegradable chelating compound [50]. In most cases, chitosan and its derivatives usages is based on their ability to chelate strongly heavy and toxic metal ions [120]. Chelation of copper and nickel by the addition of the biodegradable chelating agent, chitosan, EDTA and citrate was investigated [50]. The experiments showed that the extraction ability for copper and nickel from the contaminated soil decreased as follows: chitosan > EDTA > citrate at pH 3.00 – 3.50. Pimenta et al. [121] also found that, 0.2% chitosan, 15% EDTA and 10% citric acid gave comparable effects in decreasing dentin microhardness. Amino acids and their derivatives have been found use as chelating agents. Amino acid chelants are used to deliver minor elements to plant unlike synthetic chelates [122]. In addition, amino acids complexes of some metals are useful as; anti-inflammatory agents, antibacterial agents (as applied against Escherichia coli and streptococcus pyogenes) and anti-tumor agents (against melanoma) [123]. Furthermore, Fischer [124] investigated the ability of β-thiol group containing amino acids L-cysteine and L-penicillamine to remove heavy metals (Cd(II), Cr(III), Cu(II), Hg(II), Ni(II), Pb(II), Zn(II)) from some soil components (peat, bentonite, illite) at neutral pH. The extractability of metals from peat in the presence of L-penicillamine was slightly higher than L-cysteine in these metals. The recovery of metals from bentonite was higher generally [124]. Riri et al. [125] investigated the use of simple organic acid (oxalic, glycolic and malic acid) to chelate gadolinium (III). Lignosulfonates, proteins, humic or fulvic acids and polyflavonoids are bioderived chemicals that can be used for complexing metals and subsequent application in agricultural foliar [126-128].
Additionally, some plant extracts can also be used as chelators [129,130]. The chelating efficiency of methanolic extracts of Triticum aestivum (wheatgrass) towards iron was investigated to determine the iron chelating activity in iron dextran induced acute iron overload animals. The chelating power or efficacy of the compound was found to be 34.5% to that of desferoxamine (commercial chelant) [131]. Ebrahimzadeh et al. [132] found that the phenolic and flavonoid extract of Mellilotus arvensis has ability to chelate Fe(II) [132]. The chelating ability of aqueous extract of Tetracarpidium conophorum was tested in vitro [133]. The dose (97.38%) showed the highest chelating ability. Therefore, the aqueous extract of Tetracarpidium conophorum could be used in the treatment of iron-overload disorders due to its high chelating ability in vitro at low doses [133]. The tannin fractions isolated from hazelnuts, walnuts and almonds were characterized for chelation of Zn(II), Fe(II), Cu(II) [134]. Copper ions were most chelated by the tannin fractions of hazelnuts, walnuts and almonds. Fe(II) complexation ability of the tannin fractions of walnuts and hazelnuts were lower as compared to the almond tannin fraction [134]. The capacity to chelate Zn(II) was quite varied for the different nut tannin. An in vitro iron chelating properties of 60% ethanolic extracts of some plant parts (Terminalia chebula, Caesalpinia crista, Cajanus cajan, Terminalia belerica, Emblica officinalis, and Tinospora cordifolia) were investigated. The iron chelating property of the plant extracts as reported were; T. chebula > T. belerica > E. officinalis > C. cajan > T. cordifolia > C. crista [135].
Likewise Soya beans extract were found as chelants towards Cu(II) [136]. The binding properties of Pb(II), Cu(II), Ni(II), Cd(II), Zn(II), Cr(III) and Cr(VI) in native and NaOH-modified biomass of Solanum elaeagnifolium were investigated [137]. The result at pH 5.0 revealed that; 20.6 mg Pb(II)/ g, 13.1 mg Cu(II)/ g, 6.5 mg Ni(II)/ g, 18.9 mg Cd(II)/ g, 7.0 mg Zn(II)/ g, 2.8 mg Cr(III)/ g and 2.2 mg Cr(VI)/ g were removed respectively. Better still the NaOH modified material gave higher binding properties in each case [137]. Tsujimoto et al. [138] had observed that anacardic acids from cashew nut can chelate Fe(III). Plant extracts have been used for removal of heavy metals especially Fe [129,131,132,135,136]. They can be used in treatment of iron-overload [133] and recovery of other heavy metals from the environment [134,139]. Column experiments of 14 d and 7 d with partially hydrolyzed wool as chelating agent on a silty-loamy sand agricultural soil was studied. The 14 d wool hydrolysate mobilized 68% of Cu in soil, whereas in the case of Cd it mobilized 5.5%. The plant (Nicotiana tabacum) uptake of Cd(II) and Cu(II), assisted by the application of 6.6 g kg-1 wool hydrolysate was increased by 30% in comparison to the control plants. Phytoextraction has revealed great potential with no leaching detected unlike use of conventional chelating agents [140].
Materials and Methods
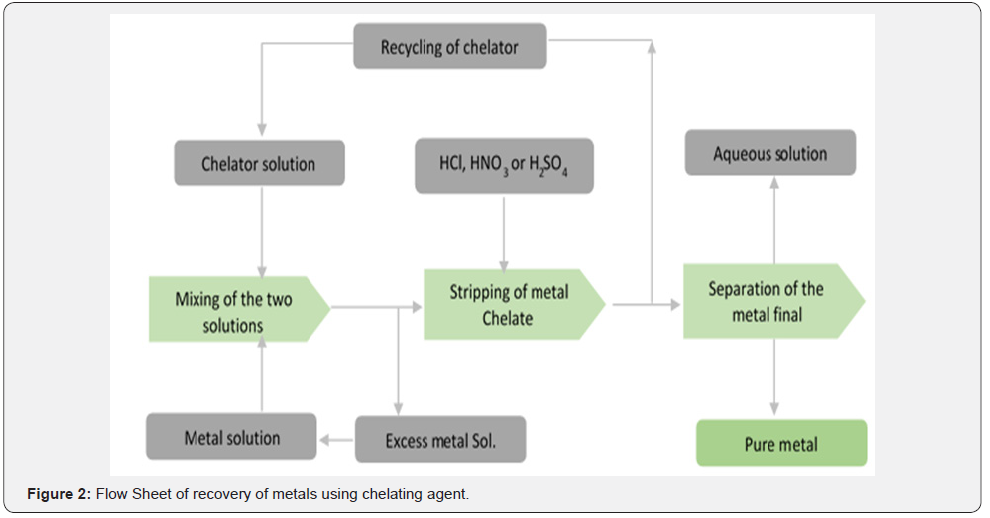
Aqueous solution of the chelant may be directly used to leach metals from spent solid waste into aqueous state. Then recovery process of metals from aqueous system with chelating agents mostly involves liquid – liquid extraction of metal ions with that of chelating agents. Solvent extraction of metals with chelating agents has been considered to be an effective method for purifying metals [141]. Subsequently the resulted complex (chelate) is stripped with strong acid (HCl or HNO3) resulting to the release of the captured metal into another aqueous phase. This is then concentrated to obtain the metal into pure state. From the literature, it has been shown that chelating agents (such APCs) alone or supported on other solids have been used for the recovery of metals [142-145]. Figure 2 is the flow sheet showing the major stages for recovery of metals using chelating agents.
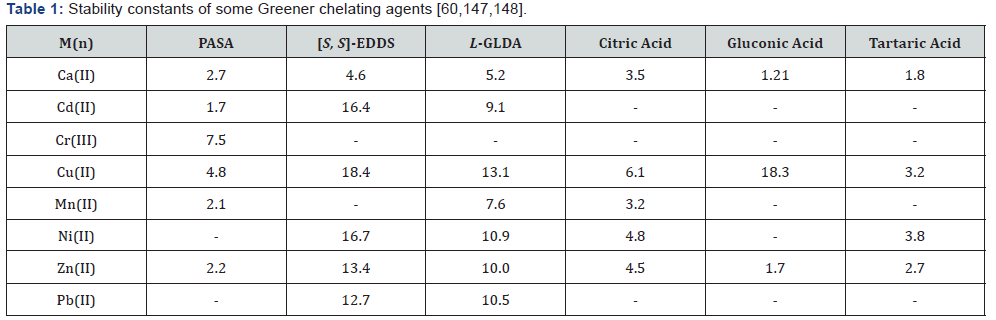
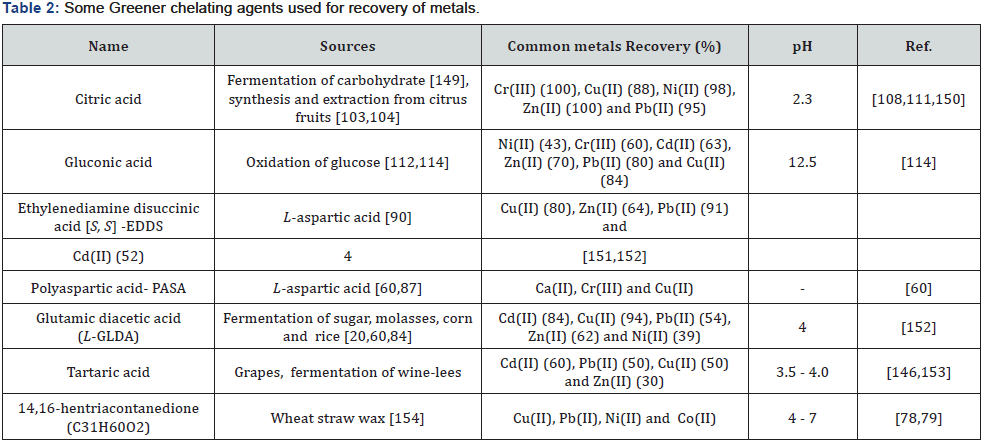
The ability of chelant to bind metal ion is determined by the stability constants [24]. Wuana et al. [146] also reported that extraction of metals with chelating agents depend on stability constants [146]. The larger the stability constant, the stronger the chelation effect and the free metal ion in solution become lesser [147]. Hence, the commonly consumed chelants (APCs) usually have high stability constants with different metal ions [24]. Table 1 gives important information because L-GLDA and [S, S]-EDDS have relatively higher stability constants for most metal ions than most other Greener chelating agents [147,148]. As a matter of fact, they have been considered as replacement for EDTA and NTA in some applications [25]. Although other factors such as temperature, pH and presence of other ions can affect the ability to remove metals by chelants [24]. Whereas, Table 2 gives some of these Greener chelants; their sources and metal chelating functions [149-154]. Again, Table 3 contain some plant extracts which have been used for removal of heavy metals especially Fe.

Materials and Methods
Classical chelating agents (especially aminopolycarboxylates, APCs and phosphonates) are till date the commonly used in industrial and home processes. This is due to their ability to strongly bind metals, and perhaps their being available in the market over a long time now. Abundant evidences proved that they are not environmentally benign. This has spurred the quest of industrialists and academia, as prompted by environmental policies, towards low toxicological profile and environmentally friendly chelating agents. The desire has resulted into annual rise in formulations and proposals of Greener alternative chelators such as glutamic diacetic acid (L-GLDA), ethylenediamine disuccinic acid [S, S]- EDDS, polyaspartic acid, citrate, gluconic acid, amino acids, lipophilic β-diketone (14,16)-hentriacontanedione, plant extracts etc. to be used in place of the classical chelants. For reasons of environmental compatibility, low toxic profile, biodegradability and sustainability, these Greener chelators are better employed for industrial and home applications. Importantly, they can be applied to recovering metals from wastes to ensure sustainability of metals and their uses.
https://juniperpublishers.com/omcij/index.php



Thank you a lot for giving everyone an extremely splendid chance to read from this blog. It's usually very nice and also packed with amusement for me personally and my office colleagues to visit the blog particularly three times in one week to read the fresh guides you have. And indeed, I am just actually contented concerning the dazzling information you serve. Selected two tips in this article are basically the simplest we have ever had.
ReplyDeleteSkin Biopsy FL