INTERNATIONAL JOURNAL OF PULMONARY & RESPIRATORY SCIENCES
Introduction
Bronchial Asthma is an airway disease with variable
degrees of bronchial mucosal inflammation and intermittent episodes of
airway obstruction and bronchial hyperesponsivness. That asthma is a
syndrome consisting of different phenotypes has been recognized for a
long time by clinicians [1]. New evidence indicates that the composition
of airway microbiota differs in states of health and disease. Different
chronic airway diseases had been related to changes in microbiota due
to various factors which could affect severity of symptoms and even
response to treatment [2]. Micro biome may be one of the protective
factors against asthma in early life [3].
What is Airway Microbiota
It a complex variety of microbes present intrachea
and different generations of the bronchi either on the mucus layer or
the epithelial surfaces or even both. These microbes include bacteria,
yeasts, viruses and bacteriophages. The bacterial part of microbiomeis
the most prevalent component with various genera: Prevotella,
Sphingomonas, Pseudomonas, Acinetobacter, Fusobacterium, Megasphaera,
Veillonella, Staphylococcus, and Streptococcus. The bronchial tree for
instance contains a mean of 2000 bacterial genomes per cm2 surface [4].
The mucosal surfaces in the human body are the home of 10-100 trillion
microbes with a diversity of greater than 1,000 species [5]. The highest
concentration of microbes is found in the GI tract, compared to those
found in the lower airways. Healthy human lungs are not sterile, as
previously believed, but it is unknown whether the microbes in the lungs
form a stable community or are a series of transient colonizers [6].
However, various theories about the origin of lower
airway microbiota in healthy individuals had been suggested. As it may
represent true colonization of the lower generations of bronchi, or it
is the result of turnover of the microbial community or it is just
contamination of oropharynx during lower airway
sampling or even linked potentially to those who are incorrectly
categorized as truly healthy [7].
Importance of microbiota
The commensal bacteria are nonpathogenic and defend our airways against the pathogens. There are several possible mechanisms:
- Commensals are the native competitors of pathogenic bacteria, because they occupy the same niche inside the human airways.
- They are able to produce antibacterial substances called bacteriocins which inhibit the growth of pathogens. Genera Bacillus, Lactobacillus, Lactococcus, Staphylococcus, Streptococcus, and Streptomyces are the main producers of bacteriocins in respiratory tract.
- Commensals are good inducers of anti allergic Th1 cascade with anti-inflammatory interleukin (IL)-10, FOXP3, and secretory immunoglobulin A (sIgA) production [7].
Airway epithelial cell and microbiota interaction
The airway epithelium together with alveolar
macrophages and dendritic cells collectively can recognize of bacterial
products trapped into the lower airways with the inhaled air. Some of
these products are can potentiate pro inflammatory stimuli. So it is a
challenging issue to distinguish between pathogens and commensals to
avoid development of constant or persistent inflammation and help to
develop tolerance against harmless microbiota [8].
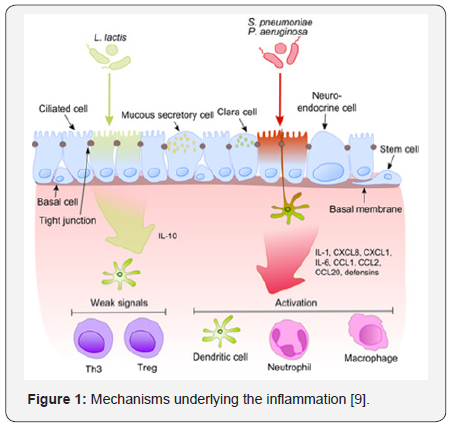
Once pathogenic bacterium (e.g., S. pneumoniae,
P.aeruginosa) has been attached to activated pattern recognition
receptors located on/in bronchial epithelial cells, the proinflammatory
cytokines pathways are predominant via release of IL-1, IL-6 and IL-8
which induce neutrophils, dendritic cells and macrophages chemotaxis to
target cells (e.g., neutrophils, dendritic cells and macrophages.
Standard microbiota fail to induce strong signaling, thus aborting
inflammation. (Figure 1) [9].
This process becomes much more intriguing when taking into
account that commensals often share their surface molecules
with pathogens. Epithelial cells are equipped with very sensitive
recognition tools - toll like receptors (TLRs), NOD like receptors
(NLRs) and retinoic acid-inducible gene (RIG)-I-like receptors
(RLRs) which determine presence of non commensal bacteria
which activate cellular components of the adaptive and innate
immunity and recruit them to the infection site [7].
NF-κB is the principal regulators of different response to
harmful microbiota as it is become activated by a number of
stimuli as bacterial cell walls or inflammatory cytokines. This
results in its translocation from the cytoplasm into the nucleus to
activate epithelial cells pro-inflammatory genes. These specific
genes can recognize a particular nucleotide sequence (5’-GGG
ACT TTC T-3’) in upstream region of response genes. [10]. Inspite
of expressing express the same microbe-associated molecular
patterns (MAMPs), harmless bacteria fails to translocate NF-κB
into the nucleus thus preventing the inflammation. The balance
between pathogens and commensals is extremely important in
the maintenance of homeostasis in the respiratory tract [9].
Pediatric acterial airway microbiota in early life
A neonatal mouse exposed to a broad-spectrum antibiotic has
been shown to increase allergen-induced airway inflammation
susceptibility [4]. Germ-free mice also exhibit enhanced airway
inflammation upon allergen exposure [3], while colonizing
OF germ free mice with microbiota from conventional mice
decreased accumulation of natural killer T (NKT) cells in their
airways .This was only observed in neonates not in adult mice.
This highlights the importance of early life as a critical period for
intervention [11].
Absence of airway colonization during this critical
neonatal window resulted in sustained susceptibility to allergicinflammation through adulthood. This ensure long-term control
of allergic airway inflammation via controlling commensal
bacteria communities early in early life [12].
Microbiota and climax community
Climax community is defined as a microbial community that
has reached a final or “climax” steady state best adapted for
growth at that specific niche along the mucosa. However, this
climax community is dynamic and still exhibits both resistance
and resilience [13]. Evidence is now accumulating that longterm
dietary pressures , repeated antibiotic use, GI illnesses or
medications such as antacids, proton pump inhibitors, and nonsteroidal
anti-inflammatory drugs can break both the resistance
and resilience of a community and result in it re-assembling into
another climax community, although this may be accompanied
by detrimental changes in host mucosal immuno biology and
physiology. One mechanism underlying the activity of probiotic
microbes and prebiotic nutrients may be the ability to restructure
a climax community to improve host mucosal immuno biology
and physiology [14].
Microbiota (microflora) hypothesis
Several theories had been suggested to explain the increase
in the incidence of asthma and other allergic diseases over the
past 30 years and the discrepancy between the higher rates of
allergic disease among industrialized relative to developing
countries. One rising assumption is a lack of early microbial
stimulation which results in aberrant immune responses to
innocuous antigens later in life “hygiene hypothesis” [15].
Life style modifications and over use of broad spectrum
antibiotics raise the concept of disturbance of mechanisms of
mucosal immunologic tolerance due to changing diversity of
gastrointestinal (GI) microbiota composition in westernized
areas [16].
Epidemiologic and clinical data supporting this interpretation include
- a positive correlation between increasing risk for asthma/allergies and increasing use antibiotics in industrialized countries,
- Altered fecal microbiota composition had been correlated to different atopic diseases
- Oral probiotics orsignificant dietary changes lead to some successful prevention/reduction of severity of allergic diseases.
Experimental data in mice compared that immune response
generation and normal ones which showed numerous defects
in immune response [17]. Altogether, these experimental,
epidemiologic, and clinical observations support the hypothesis
that even minor changes in the quality or quantity of airway
microbiota can be one of the predisposing factors for allergic
disease [10].
Cross-talk between the gut and the lung
The existence of the gut–lung axis and its implications
for airway disease provide a portal for potential therapeutic
intervention in prevention or management of asthma [18].
Oral supplementation with probiotic strain of Bifidobacterium
and prebiotic non-digestible oligosaccharides reduced airway
IL6 and IL4 levels and protected against HDM-induced airway
inflammation. This suggest that some intestinal bacteria have
the capacity to suppress inflammation at a distal mucosal site
[19].
Oral tolerance and airway tolerance
Oral tolerance is defined as the propensity of ingested
antigens to abort subsequent systemic immune responses.
Gastrointestinal tract may be also involved in tolerance to inhaled
and ingested antigensvia CD4+ regulatory T cells (Tregs) that
produce immunosuppressive cytokines, IL-10 and TGFβ, in what
is termed “bystander suppression.” [19,20]. Mucosal signals,
such as those from the microbiota, keep resident dendritic cells
in an immature or non-inflammatory state [15].
Airway microbiata diversity in asthma
In asthmatic patients, certain airway microbial composition
was associated with airway eosinophilia and AHR to mannitol
but not airway neutrophilia. Comparing eosinophilic and
noneosinophilic asthmaas regards airway microbiome revealed
that Asthmatic patients with the lowest levels of eosinophils
had an altered bacterial microbial profile, with more Neisseria,
Bacteroides, and Rothia species and less Sphingomonas,
Halomonas, and Aeribacillus species compared with asthmatic
patients with high eosinophilia. This may invite furtherresearch
on effect of modulating diversity of microbiota to modulate
various asthma phenotypes [21].
Airway microbiota dysbiosis in asthma
Airway dysbiosis in patients with severe asthma appears to
differ from that observed in those with milder asthma. Specific
Bacterial communities as Proteobacteria were associated with
worsening ACQ scores and sputum total leukocyte values in
severe and poorly controlled asthma. Actinobacteria had been
associated with stable or even improving ACQ scores and can
predict steroid responsiveness [22].
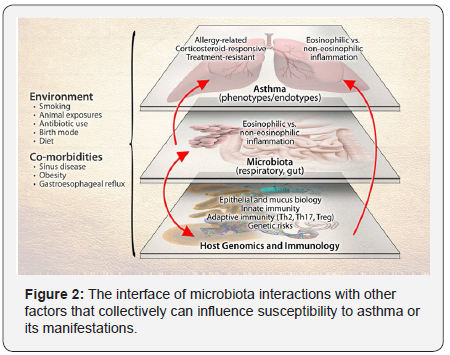
Airway microbiota and asthma heterogeneity
Dissecting the role of the microbiome in asthma is challenged
by the heterogeneity of the disease at multiple levels (Figure
2). These levels include asthma’s clinical and inflammatory
heterogeneity, genetic factors that contribute to asthma risk, and
the multiplicity of immune pathways involved in asthma. The
potential effects of environmental exposures on gene function,
immune responses, as well as microbiota composition add further
complexity. As with genetics, mechanistic consequences of the
altered microbiome may explain certain aspects or phenotypes
of asthma as the development of allergic or non-allergic asthma,and treatment-resistant asthma) [22] Components of the
depicted system-host genetics and immunology, microbiota,
environmental exposures, and the disease of asthma- are
themselves heterogeneous entities, presenting challenges to
more precisely dissect the role(s) of the microbiome in asthma.
Upper airway microbiota and asthma
Bisgaard et al. [23] demonstrated that the nasopharyngeal
microbiome composition was influenced by the early life
exposures, including attending day care, having siblings, and
taking antibiotics. Haemophilus, Streptococcus, Moraxella had
been previously associated with airway disease and increased
risk for asthma exacerbations. Early colonization with either
Moraxella, or Streptococcus was strongly associated with acute
lower respiratory viral infections. This colonization can be
predictor for asthma development later in life.
Thus, probiotic intervention studies of animals provide
encouraging evidence for intentional manipulation of the
intestinal microbiota as a strategy for asthma prevention and
management. A meta-analysis of a large number of randomized
trials of probiotic supplementation, on atopic sensitization
and asthma in children, however, shows that the success of
these interventions in mice does not translate easily to disease
prevention in humans. At a minimum, this highlights that
different probiotics may have distinct interactions with the host
microbiome and that some strains might be more specific for
modulating atopic inflammation but many other considerations,
such as diet, age of intervention, coincident environmental
exposures, length of supplementation period, and other as yet
unknown factors, are likely important [24].
Airway microbiota and severity of asthma
Relationships between the airway microbiome and disease
features have also been examined in patients with in severe
asthma. Different clinical phenotypes of severe asthma have
been described, suggesting the possible involvement of alternate
mechanistic pathways, as has been surmised for asthma in general. A preliminary analysis of the bronchial microbiome in
these subjects, poorly controlled despite high-dose ICS therapy,
noted significant relationships between different bacterial
community profiles and features such as body-mass index and
measures of asthma control [25]. A similar study of sputum
bacterial composition in 28 treatment-resistant asthmatics
found that the relative abundance of M. catarrhalis, Haemophilus,
or Streptococcus spp. correlated with worse lung function and
higher sputum neutrophil counts and IL-8 concentrations [19].
Microbiota and therapy of allergic disease
The composition of the microbiota can be manipulated by
combinations of antibiotics, probiotics, and dietary components
which may have direct growth promoting or inhibiting activity
for specific microbes. [26]. Certain types of fatty acids, phenolic
compounds, and carbohydrates may modulate these microbiota.
However, a single type of probiotic or dietary component will
not be efficacious in all individuals. This likely due to differences
in the types of microbial communities in different individuals.
The objective of the international Human Microbiome Project
is to characterize and define the human microbiome in states
of health and disease [10]. The challenge for future research is
to use this information to optimize probiotic/dietary therapy
to improve human health and prevent microbiota-associated
diseases, such as allergies .They are likely to include short chain
fatty acids and ionic polysaccharides [27] .
Microbiota and prevention of allergic disease
Probiotic intervention studies of animals provide
encouraging evidence for intentional manipulation of the
intestinal microbiota as a strategy for asthma prevention and
management. However, A large number of randomized trials on
the value of probiotic supplementation, on asthma incidence and
severity in children, could not show the same success of these
interventions as in mice [28-30]. This may be due to many other
considerations, such as diet, age of intervention, coincident
environmental exposures, length of supplementation period,
and other as yet unknown factors, are likely important [24,31-
34].
To Know More About International journal of pulmonary & Respiratory Sciences Please click on:
To Know More About Open Access Journals Please click on: ttps://juniperpublishers.com/index.php




No comments:
Post a Comment