Cancer Therapy & Oncology - Juniper Publishers
Abstract
Germ cell neoplasia in situ emerges as a precursor lesion configuring type II germ cell tumors as testicular seminoma or post-pubertal non-seminoma Tous testicular germ cell tumors. Lesion is comprised of neoplastic gonocyte-like cells, latent totipotent or naive germ cells with developmental potential situated within ‘spermatogonia niche’ of seminiferous tubules. Germ cell neoplasia in situ delineates an increased incidence with conditions such as uncorrected cryptorchidism, ambiguous genitalia, infertility or preceding history of post-pubertal germ cell tumor within contralateral testis. Neoplasm demonstrates aneuploidy or polypoid genotype with additional chromosomal gains as isochromosome 12p upon commencement of neoplastic invasion. Neoplastic cells appear enlarged, atypical, gonocyte-like and are incorporated with abundant, clear cytoplasm, enlarged, hyperchromatic nuclei, coarse nuclear chromatin, angulated cellular margins and prominent nucleoli.
Keywords: Precursor lesion; Primordial germ cells; Aneuploidy; Bilateral microlithiasis; Chemotherapy
Abbreviations: WHO: World Health Organization; GCNIS: Germ Cell Neoplasia in Situ; UNL: Upper Normal Limit; LDH: Lactic Dehydrogenase; FISH: Fluorescent in Situ Hybridization; SNP: Single Nucleotide Polymorphism; PLAP: Placental Alkaline Phosphatase; AFP: α fetoprotein
Introduction
Germ cell neoplasia in situ is a commonly discerned precursor lesion configuring type II germ cell tumors as testicular seminoma or post-pubertal non-seminomatous testicular germ cell tumors. Lesion is comprised of neoplastic gonocyte-like cells and latent totipotent or naive germ cells with developmental potential which are situated within ‘spermatogonial niche’ of seminiferous tubules. As per current World Health Organization (WHO) classification, neoplasm is designated as germ cell neoplasia in situ and represents as a precursor for a subset of adult germ cell tumors. Alternative terminology as intra-tubular germ cell neoplasia, intra-tubular germ cell neoplasia unclassified subtype, carcinoma in situ testis or malignant germ cells is not recommended. Appropriate discernment of germ cell neoplasia in situ may be challenging, especially in pediatric subjects or individuals demonstrating infantile or pre-pubertal testis on account of morphological concurrence and immunohistochemistry concordant with normal or delayed maturation of testis. Surgical sampling of a testicle from subjects incriminated with extra-gonadal germ cell tumor may represent with ‘burnt out’ germ cell tumor.
Germ cell neoplasia in situ expounds an increased incidence with associated conditions such as uncorrected cryptorchidism, ambiguous genitalia, infertility, or preceding history of post-pubertal germ cell tumor within contralateral testis. Frequently, lesion is accompanied by aneuploidy although lack of isochromosome 12p appears within invasive adult germ cell tumours [1,2]. Molecular assay of neoplastic cells demonstrates aneuploidy or a polypoid genotype with additional chromosomal gains occurring upon commencement of neoplastic invasion, as enunciated with isochromosome 12p discerned within invasive disease and absent within germ cell neoplasia in situ [1,2]. Frequently, germ cell neoplasia in situ arises within testicular seminiferous tubules incriminated with post-pubertal germ cell tumours and occasionally represents as a residual manifestation or ‘burnt out’ germ cell tumour [2,3]. Germ cell neoplasia in situ is posited to arise from incompletely differentiated primordial germ cells which expound whole genome duplication events with subsequent repetitive loss of chromosomal arms or entire chromosomes. Therefore, an aneuploidy within the neoplastic configuration ensues with subsequent emergence of additional chromosomal mutations within a subset of tumefaction as genetic mutations within KIT gene or KRAS gene [2,3].
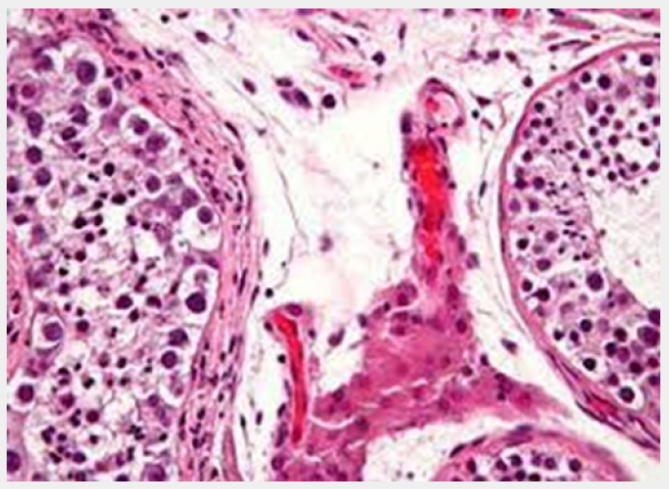
Preliminary genomic mutations within KIT gene following genome duplication may engender a subset of testicular seminoma comprehensively delineating genomic hypomethylation. Besides, differentiation into diverse histological subtypes is absent. Germ cell neoplasia in situ is postulated to manifest as a ubiquitous precursor of type II germ cell tumours which represent ~95% of germ cell tumours arising within post-pubertal males as testicular seminoma, embryonal carcinoma, choriocarcinoma, testicular teratoma or yolk sac tumour [2,3]. Overexpression of embryonic transcription factors may elevate cellular proliferation and abolish apoptosis. Germ cell neoplasia in situ is comprehensively encountered within testicular seminiferous tubules incriminated with adult germ cell tumours. Occasionally, contralateral testis may exhibit foci of germ cell neoplasia in situ. Additionally, lesion may concur as a residual feature with ‘burnt out’ adult germ cell tumour [3,4]. Upon microscopy, neoplastic cells appear confined to basement membrane of seminiferous tubules and configure a designated ‘spermatogonial niche’ [3,4]. Neoplastic cells appear enlarged, atypical and gonocyte-like and are incorporated with abundant, clear cytoplasm, enlarged, hyperchromatic nuclei, coarse nuclear chromatin, angulated cellular margins and prominent nucleoli, reminiscent of seminoma cells. Tumour cell nuclei appear up to 11 μ metres in diameter. Frequently, incriminated seminiferous tubules delineate a thickened basement membrane, peritubular hyalinization and lack of spermatogenic maturation. Tumefaction demonstrates a ‘pagetoid’ pattern of neoplastic dissemination into rete testis or a plane between epithelium layering rete testis and basement membrane [3,4] (Figures 1 & 2).

TNM staging of carcinoma testis [3,4]
Primary tumour
i. TX Primary tumour cannot be assessed.
ii. Tis Germ cell neoplasia in situ (GCNIS).
iii. T0 No evidence of primary tumour within the testis.
iv. T1 Primary tumour confined to testis and rete testis.
Vascular or lymphatic infiltration is absent. Tunica albuginea is invaded. Tumour invasion into tunica vaginalis is absent.
Pure seminoma is subdivided as:
i. ~T1a Tumour magnitude < 3 centimetres.
ii. ~T1b Tumour magnitude ≥ 3 centimetres.
iii. T2 Tumour confined to testis, rete testis and extends into ≥ one components of testis as blood vessels, lymphatics, epididymis, adipose tissue confined to hilar soft tissue adjacent to epididymis or tunica vaginalis.
iv. T3 Tumour extends into spermatic cord.
v. T4 Tumour extends into scrotum.

Regional lymph nodes
Clinical staging of regional lymph nodes is assessed with imaging techniques such as computerized tomography(cN).
Pathological staging of regional lymph nodes is assessed with dissection of regional, retroperitoneal, para-aortic, peri-aortic, inter-aortocaval, paracaval, pre-aortic, precaval, retro-aortic and retrocaval lymph nodes(pN).
i. NX Regional lymph nodes cannot be assessed.
ii. N0 Regional lymph node metastasis absent.
iii. N1 Regional lymph node metastasis confined to one to five retroperitoneal lymph nodes with magnitude < 2 centimeters.
iv. N2 Regional lymph node metastasis into minimally a singular enlarged lymph node or lymph node mass >2 centimeter and <5-centimetre diameter OR metastasis into >5 regional lymph nodes <5-centimeter diameter OR metastasis into minimally a singular lymph node between 2 centimeter and 5-centimeter diameter.
v. N3 Regional lymph node metastasis into minimally a singular enlarged retroperitoneal lymph node or lymph node mass > 5-centimeter magnitude OR metastasis into minimally a singular enlarged lymph node or lymph node mass > 5-centimeter diameter.
Distant Metastasis
i. MX Distant metastasis cannot be assessed.
ii. M0 Distant metastasis into distant lymph nodes or various organs absent.
iii. M1 Distant metastasis into:
a. ~M1a Metastasis into pulmonary parenchyma or distant lymph nodes as pelvic, thoracic, supraclavicular or visceral lymph nodes apart from retroperitoneal lymph nodes.
b. ~M1b Distant metastasis into viscera as hepatic parenchyma, skeletal system or brain. Pulmonary parenchyma may or may not be incriminated.
Serum Tumour Markers
i. SX Serum tumour marker levels unavailable.
ii. S0 Serum tumour marker levels appear normal.
iii. S1 Minimally a singular tumour marker level exceeds normal range as
a. ~lactic dehydrogenase (LDH) <1.5 times upper normal limit (ULN)
b. ~βHCG < 5,000 mIu/mL.
c. ~alpha fetoprotein (AFP) <1,000 ng/mL.
iv. S2 Minimally a singular tumour marker appears substantially above normal range as.
a. ~lactic dehydrogenase (LDH) between 1.5 times to 10 times upper normal limit (ULN).
b. ~βHCG between 5,000 to 50,000 mIu/mL.
c. ~alpha fetoprotein (AFP) between 1,000 to 10,000 ng/ mL.
v. S3 Minimally ≥ one or more tumour markers are significantly elevated.
a. ~lactic dehydrogenase (LDH) > 10 times upper normal limit (ULN).
b. ~βHCG > 50,000 mIu/mL
c. ~alpha fetoprotein (AFP)> 10,000 ng/mL
Discussion
Tumour cells appear immune reactive to OCT3/4, SALL4, podoplanin or D2-40, SOX17, NANOG, LIN28A, AP2 gamma, placental alkaline phosphatase (PLAP) and c-KIT or CD117. Tumor cells appear immune non-reactive to α-inhibin, SF1, Wilm’s tumor 1(WT1) antigen, pan-cytokeratin AE1/AE3, CD30, SOX2, α fetoprotein (AFP) or glycpican 3 [5,6]. Germ cell neoplasia in situ requires segregation from neoplasms such as infantile or prepubertal testis with gonocytes or normal or delayed testicular maturation, intratubular seminoma or seminoma with microinvasion [5,6]. Fluorescent in situ hybridization (FISH), single nucleotide polymorphism (SNP) array or genetic karyotyping may be beneficially adopted to detect aneuploidy or absence of isochromosome 12p. Germ cell neoplasia in situ can be appropriately ascertained with precise histological examination of surgical tissue samples as testicular biopsy or orchiectomy. Alternatively, tissue sampling of contralateral testis incriminated with adult germ cell tumor may be beneficially adopted. Neoplasm is devoid of specific serum biomarkers or pertinent features in imaging. Upon ultrasonography of subjects demonstrating infertility, unilateral or bilateral microlithiasis may be discerned, a feature which is associated with enhanced incidence of germ cell neoplasia in situ [5,6].
Surgical tissue sampling of testis may be adopted in subjects delineating microlithiasis along with minimally a singular, concurrent condition as uncorrected cryptorchidism, ambiguous genitalia, infertility or preceding history of post-pubertal germ cell tumour within contralateral testis [5,6]. Germ cell neoplasia in situ can be appropriately treated with orchiectomy. Besides, extensive surveillance is recommended to evaluate neoplastic progression. Employment of adjuvant chemotherapy appears non concurrent with decimation of risk of disease progression. Low dose radiotherapy may be contemplated for alleviating germ cell neoplasia in situ within contralateral testis in subjects delineating localized adult germ cell tumour. An estimated ~50% of neoplasms progress into invasive adult germ cell tumour within 5 years wherein ~90% tumours evolve into invasive adult germ cell neoplasia within 7 years [5,6].
Conclusion
Tumour cells appear immune reactive to OCT3/4, SALL4, podoplanin or D2-40, SOX17, NANOG, LIN28A, AP2 gamma, placental alkaline phosphatase (PLAP) and CD117. Neoplastic cells appear immune non-reactive to α-inhibin, SF1, Wilm’s tumour 1(WT1) antigen, pan-cytokeratin AE1/AE3, CD30, SOX2, α fetoprotein (AFP) or glycpican 3. Germ cell neoplasia in situ requires segregation from neoplasms such as infantile or prepubertal testis with gonocytes or normal or delayed testicular maturation, intratubular seminoma or seminoma with microinvasion. Germ cell neoplasia in situ can be appropriately treated with orchiectomy.
To Know more about Cancer Therapy & Oncology International Journal
Click here: https://juniperpublishers.com/ctoij/index.php
To Know more about our Juniper Publishers
Click here: https://juniperpublishers.com/index.php




No comments:
Post a Comment