Nanomedicine - Juniper Publishers
Abstract
The present study aimed at the biologically and chemically synthesized copper oxide nanoparticles as biosensors for melatonin hormone. Methanol extracted Hyptis suaveolens is used as a reducing agent for the biological synthesis of CuO nanoparticles. The synthesized copper oxide nanoparticles were characterized by using UV-Vis spectroscopy, Fourier transform infrared spectroscopy, Scanning electron microscopy, Energy dispersive X-ray analysis, and X-ray diffraction. UV –Vis absorbance of biologically and chemically synthesized CuO Nanoparticles was absorbed in a wavelength range of 249 nm and 264 nm, indicating the formation of CuO Nanoparticles. In Fourier Transform Infrared Spectroscopy (FT – IR) observed functional groups are phenolic compounds, amines, carboxylic group, amines, aliphatic amines, alkaloids, alkanes. SEM and EDAX analysis are in clear and uniform size, almost in spherical in nature and the element composition of CuO Nanoparticles were 82 %, 67% by using Energy Dispersive X-ray Spectroscopy. X-ray diffraction (XRD) peaks are indexed according to the hexagonal phase and a good spherical shape was found as 65 nm and biologically and chemically synthesized CuO Nanoparticles were used for the detection of melatonin hormone in a serum sample to study electrochemical sensing properties and in pH 12. The study concludes that the biologically synthesized CuO Nanoparticles are high potential than chemically synthesized CuO Nanoparticles.
Keywords:Biological; Chemical; Copper oxide; Nanoparticles; Biosensor; Melatonin hormone
Introduction
Nanoparticles have a positive impact in improving many sectors of the economy including consumer products, energy, transportation, cosmetics, pharmaceuticals, antimicrobial agents and agriculture. The composites prepared using metal nanoparticles and polymers can find better utilization due to the enhanced antimicrobial activity. Nanoparticles play a vital role in the production of food products against microbial contamination. It also acts as carrier vehicles of nutrients, nutraceuticals, enzymes, food additives, and also improves the physical, chemical and nutritional quality of feed and its respective ingredients by application of nanotechnology in the different steps for manufacturing [1]. Generally, the nanoparticles are synthesized by physical, chemical and biological by using top-down and bottomup strategies [2]. Among the different methods, green technology is an emerging and powerful technology for the development of innovative, reliable and sustainable solutions to address global issues through research, development and innovation.
Biosynthesis strategies show desirable characteristics under the sustainability perspective that are eco-friendly and are simple and rapid to produce biocompatible, biodegradable, low cost and high yield products [3]. The biologically synthesized nanoparticles are unique and have physicochemical, optical and biological properties, which can be manipulated suitably for desired applications. Biosynthesis methods are more efficient than other classical synthesis procedures. Recently the biologically synthesized nanomaterials have been found to have potential applications in different areas such as treatment, diagnosis, development of surgical Nano devices and commercial product manufacturing. Nano medicine makes a huge impact on the health care sector in treating various chronic diseases.
Some of the nanoparticles such as silver, titanium dioxide, zinc oxide and copper oxide are receiving considerable attention as antimicrobials and additives in consumer, health-related and industrial products. Among the numerous metal oxide nanoparticles, copper oxide has attracted significant attention because it can be used as an antimicrobial, antibiotic and antifungal agent when incorporated in coatings, plastics and textiles etc. [4]. Copper oxide nanoparticles are used to develop a good sensor, because of their highly specific area, good electrochemical activity and promoting the electron transfer reaction at a lower voltage and hence used in gas sensors, photovoltaic cells, in catalyst applications and in heat transfer Nano fluids. The biologically and chemically synthesized copper oxide nanoparticles have electrochemical properties, which can be used in the field of medicine, fish farming and fabricating biosensors. The present study aimed at the biologically and chemically synthesized copper oxide nanoparticles as biosensors for melatonin hormone.
Materials and Methods
Biosynthesis of CuO nanoparticles
The precipitation method was adopted for the synthesis of copper oxide nanoparticles. Methanol extracted Hyptis suovelens (L) Poit leaf extract and CuSO45H2O in the ratio 1:1 and 1mM of 100ml of copper sulphate was used under stirring vigorously in magnetic stirrer for 2 minutes. After stirring the precipitation was achieved. The pH was observed at 7 and kept overnight. Then the CuO precipitate was centrifuged at 3500 rpm for 10 minutes. The centrifugal process continued with water and repeated the centrifuging process for purifying CuO. Then the precipitate was dried at room temperature. Finally, copper oxide nanoparticles were stored in dry tube containers.
Chemical Synthesis of CuO nanoparticles
The CuO nanoparticles were synthesized by a simple chemical reduction method using copper (II) sulphate pentahydrate (CuSO4.5H2O) as precursor and starch as capping agent. The synthesis was started with the addition of 0.1 M CuSO4.5H2O solution into 120 ml of starch (1.2%) solution with constant stirring under a magnetic stirrer for about 30 min. Then 50ml of 0.2 M ascorbic acid solution is added under continuous rapid stirring. Subsequently, 30 ml of 1M sodium hydroxide solution was slowly added to the prepared solution with constant stirring and heating at 80°C for 2 hrs. The colour of the solution turned yellow to Ochre. After the completion of the reaction, the solution was taken from the heat and allowed to settle overnight and the supernatant solution was discarded. Then precipitates were separated by filtration and the filtrate was washed with deionised water and ethanol three times to remove the excess starch which is bound with nanoparticles. Ochre colour precipitates obtained was dried at room temperature. After drying, nanoparticles were obtained and stored in a glass vial for further studies.
Characterization
The synthesized copper oxide nanoparticles were characterized by the following techniques.
UV- Visible Spectroscopy.
The formation of CuO NPs was initially confirmed visually and by using UV-visible spectroscopy technique, which has been frequently used to characterize the synthesized metal and metal oxide nanoparticles.
Fourier Transform Infrared Spectroscopy (FTIR)
The FTIR analysis was used to identify the capping, reducing and stabilizing capacity. The FTIR spectra of synthesized copper oxide nanoparticles were analyzed for knowing the possible functional groups. The measurement was carried out by JASCO (FTIR- 6200) spectrum.
SEM and EDAX Analysis
The surface morphology and size of the nanoparticles were obtained by Scanning Electron Microscopy (SEM) analysis. The morphology and composition of the copper nanoparticles were examined by Scanning Electron Microscope (SEM) using HITACHI SU600 equipped with energy-dispersive.
X-ray Diffraction Analysis
X-ray diffraction (XRD) analysis was carried out by a powder diffractometer (Rigaku III/A, Japan) equipped with a copper oxide target and a nickel filter to analyze the structure of the product.
Biosensor
A biosensor could be a device that uses a living organism or biological molecules, particularly enzymes or antibodies, to observe the presence of chemicals. Melatonin is secreted by the pineal gland, exhibits strong antioxidant activity and helps to regulate other hormones, maintaining the body’s circadian rhythm. Numerous investigations have suggested that low melatonin levels may be associated with risks of breast cancer, prostate cancer type 2 diabetes and supplements may help sufferers of insomnia and menopause. The measurement of urinary melatonin is also useful for monitoring levels of serum melatonin following administration. The reference concentration range of melatonin in urine is reported to be 2.0–35.4 pg/ ml.
Preparation of sample
Both biologically and chemically synthesized CuO Nps were dissolved in 1mg /ml and sonicated for 20 min for further experimental work.
Preparation of the Modified Electrodes
Preparation of the modified electrodes GCE was polished before each experiment with 1, 0.3 and 0.05g alumina powder (CHI Instrument, Shanghai, China) in sequence, rinsed thoroughly with double distilled water between each polishing step, ultrasonicated in 1:1 HNO3, ethanol, and double-distilled water, and allowed to dry at room temperature. Then 1.0 mg functionalized with carboxylic acid groups was dispersed in 1.0 ml of 0.5%. For the chemical synthesis of CuO Nps, GCEs were modified by a 4 drop of CuO solution and dried in air. The CuO Nps was electrochemically deposited on the electrodes under a multicyclic voltammeter in 0.1 M Na2SO4 + 2.0 mM CuSO4 deoxygenated by high purity nitrogen for 10 min. The cyclic number and the scan rate of the potential scanning were optimized as 30 cycles at 100 mV s-1 for obtaining high sensitivity and reproducibility of the electrodes. The electrodes were rinsed with water, modified by 4 drops of 0.5% on the surface, dried and stored at 4oC in a refrigerator.
Experimental measurements
The experiments were conducted at room temperature in a conventional electrochemical cell. The active surface area was estimated by steady-state voltameter in a solution of 20 mM K4Fe (CN) 6 with 0.2 M KCl as the supporting electrolyte. The electrochemical impedance spectroscopy (EIS) measurements were also performed in 10 mM [Fe (CN) 6] 3/4 solution containing 0.1 M KCl and plotted in the form of complex plane diagrams and were recorded with a frequency range of 0.01 Hz to 10 kHz. The amplitude of the applied sine wave potential is 5 mV, with a formal potential of 0.143 V. Cyclic voltammetry (CV) experiments were carried out in quiescent solution with a scan rate of 50 mV s -1 in an electrochemical cell filled with 5.0 ml of 20 mM NaOH. The current–time curves were recorded at 0.65 V under stirring.
Results and Discussion
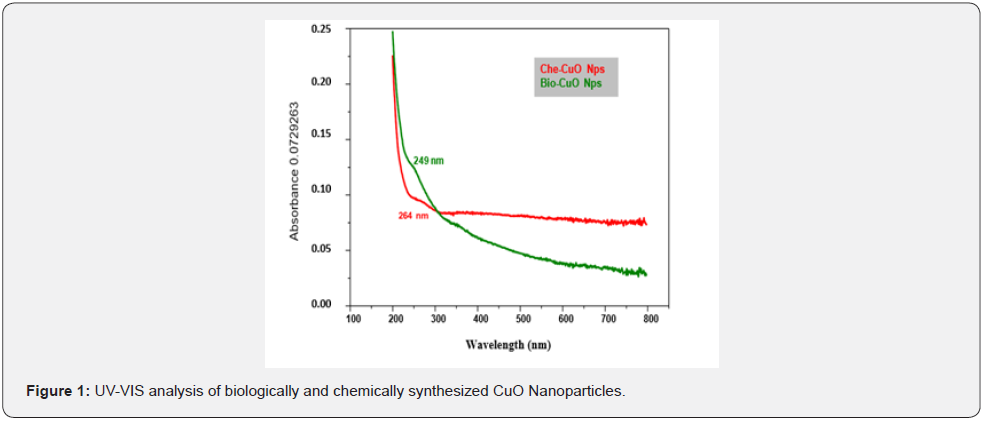
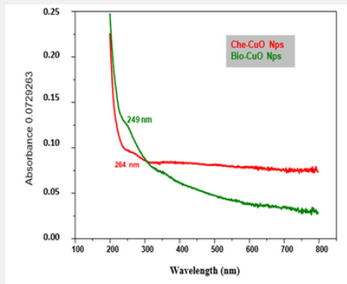
The UV - Vis analysis of biologically and chemically synthesized CuO Nps shows the sharp peaks at 249nm and 264nm, this peak indicates the formation of CuO NPs (Figure 1). The biologically synthesized CuO nanoparticles by using Phyllanthus amarus leaf extract and UV-spectra indicated that the CuO SPR bands are obtained around 285 nm with the absorption edge at 436 nm [5]. Similarly, a strong absorption peak at 269 nm for CuO Nps synthesized by using tea leaf and coffee extraction and between 200 – 300nm for CuO Nps synthesized from C. Papaya leaves extract [6]. Also reported that the synthesis of copper oxide nanoparticles (CuO Nps) using Gloriosa superba L. plant extract and UV–vis spectra, the formed CuO Nps dispersed in water exhibiting the maximum absorption peaks at about 380 nm [4]. The synthesized copper oxide particles are subjected to UV- spectroscopic analysis and obtained a single peak but broad at 263nm indicating the presence of oxides of copper metal [7] UV-Spectrum of copper nanoparticles from Nerium oleander leaf aqueous extract is in the range 250-450 nm [8] Also reported the synthesis of copper oxide nanoparticles (CuO NPs) using fruit extract of Syzygium alternifolium, peak manifested at 285 nm in UV–Vis analysis confirms the synthesis of CuO NPs [9].
The FT-IR spectra of the Hyptis suveolens (L) poit methanol extract absorbed in different peaks (Figure 2). In biologically synthesized copper oxide nanoparticles, peak values at 3947.47, 3407.04, 2924.51, 1610.08, 1276.10, 1107.36, 817.95, and 616.56cm-1 (Figure 2a) were observed. The peak at 616.56 cm-1and 3947.47cm-1 corresponds to O-H stretch phenolic compounds, N-H stretch of amines, O-H stretch of Carboxylic group, N-H bending of amines, C-H stretch of aliphatic amines, C-Cl stretch of alkaloids and C-H bending of alkanes. In chemical synthesis, copper oxide nanoparticles were carried out to identify the possible functional groups responsible for the reduction of the copper Oxide Nps (Figure 2b). The bands are observed in the sample at 608.07, 1027.54, 1375.48,1108.019,1009.96, 1108.01, 1634.39, 617.07, 1018.60, 1411.25, 1099.07, 1625.45 and 617.01 cm-1 were associated with C-O alcohol, N=O Nitro group, C= CO Carbonyl and C-CL alkaline Halide. In biologically and chemically synthesized copper oxide nanoparticles, FT- IR peaks correspond to O-H stretch phenolic compounds, N-H stretch of amines, O-H stretch of Carboxylic group, N-H bending of amines, C-H stretch of aliphatic amines, C-Cl stretch of alkaloids, C-H bending of alkanes and these peaks are similarly reported [10].
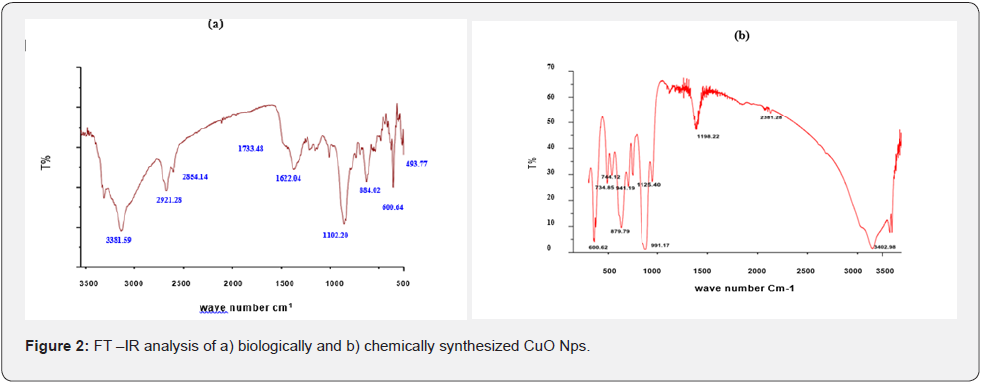
A peak at C-H bending of alkanes (622cm-1) indicates the formation of cuprous oxide nanoparticles synthesized by using Camellia sinensis (Green tea) plant extract [11]. The bands around 3450, 1600 and 2350 cm−1 show the presence of O-H, C–C and C=O stretching of hydroxyl groups, alkenes and presence of alkanes respectively, a strong band at 1100 cm−1, and 529, 350 cm−1 confirming the formation of highly pure CuO nanoparticles. Also reported spectral peaks proposing the occurrence of bands relevant to amide NAH stretching (3444 cm-1), alkane CAH stretching (2926 cm-1) anhydride C-O bending (1880 cm-1 ) and C-O stretching (1087 cm-1 )[5] The existence of band at 473 cm-1 recognized as O-H stretch of Carboxylic group, confirming the formation of copper oxide nanoparticles [12].
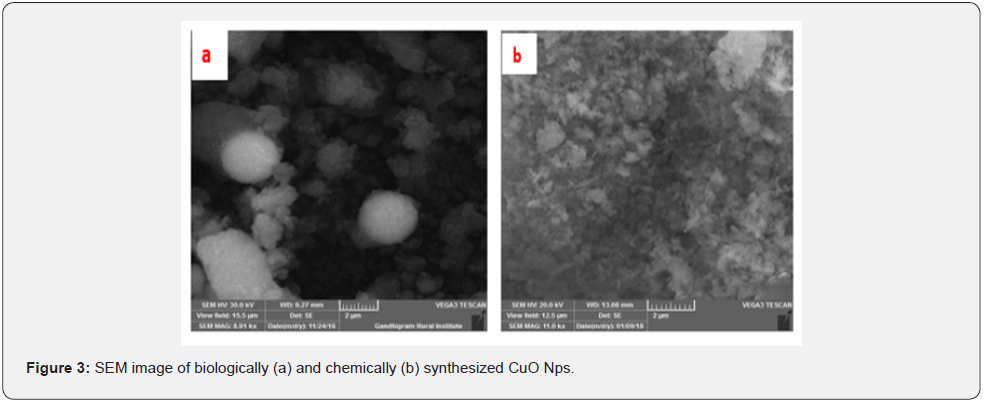
The SEM images of biologically and chemically synthesized CuO Nps are presented in (Figures 3a & 3b). It clearly shows that the particles are small and uniform in size, almost spherical in nature which is free from agglomeration in the biologically synthesized copper oxide nanoparticles. Several researchers also reported that the copper nanoparticle was synthesized by using the extract of Erzincan Cimini grape Vitis viniferacv, Aloe barbadensis, Capparis zeylanica Gultekin and Aloe vera flowers extract [13- 16]. reported that the formation of copper nanoparticles from the as well as their morphological dimensions in the SEM study demonstrated that the average size was 40 nm and was spherical in shape [17] The SEM image of tea decoction stabilized copper nanoparticles prepared from copper sulfate salt and the image show clear spherical morphology with an average particle size around 5 nm [18]. Highly crystalline CuO nanoparticles (NPs) using oak fruit hull (Jaft) as reducing and stabilizing agents were also reported [19].
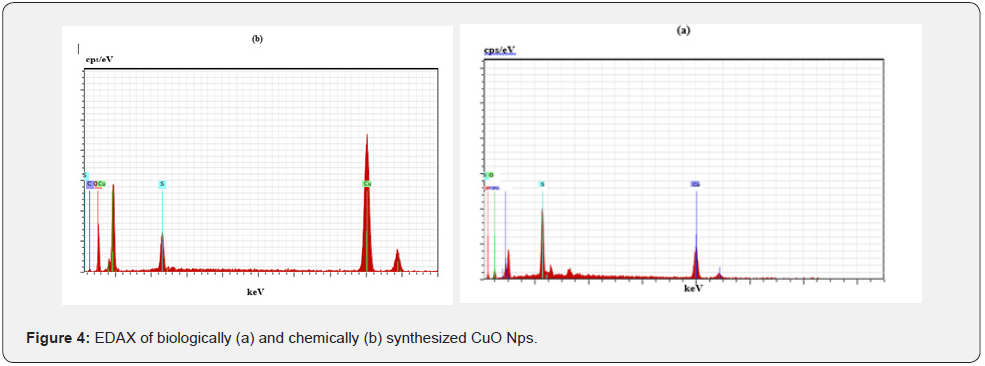
The elemental composition of the biological and chemical synthesized CuO Nps are identified (Figures 4a & b) and were 82 % and 67% by an Energy Dispersive X-ray Spectroscopy. The synthesized copper oxide nanoparticles (CuO NPs) using fruit extract of Syzygium alternifolium and the EDX analysis of nanoparticles showed 34.32 and 31.54% of copper oxide [9]. The elemental composition of the biological and chemical synthesized CuO Nps was identified as spherical in nature and sizes were 82 % and 67% by an Energy Dispersive X-ray Spectroscopy. The presence of copper and oxygen signal peaks in the EDX spectrum confirms CuO Nps which are synthesized by using Momordica charantia leaf extract [20]. Also reported that Aloe barbadensis mediated copper oxide nanoparticles were spherical in nature and elemental compositions were confirmed by EDX [21].

XRD patterns of biologically and chemically synthesized CuO nanoparticles are presented in (Figure 5). In biosynthesized CuO Nps (Figure 6a) a series of diffraction peaks at 2θ of 32.55˚, 35.56˚, 38.75˚, 48.75˚, 53.48˚, 58.34˚, 61.57˚, 65.43˚, 66.27˚, 72.40˚ and 75. 25˚ were assigned to (110), (111), (200), (−202), (020), (202), (−113), (-311), (022), (311) and (004) planes respectively, which are in good agreement with those of powder CuO Nps obtained from the International Centre of Diffraction Data card (JCPDS-80-1916). In chemical synthesized CuO Nps, the XRD diffraction peaks are indexed as 34.014˚ (002), 56.523˚ (111), 69.234˚ (204), 67.002˚ (202) and 68.393˚ (113) (Figure 6b). All diffraction peaks are indexed according to the hexagonal phase of copper oxide nanoparticles ((JCPDS- 80-1916) and no characteristic peak impurity phase except copper oxide nanoparticles and revealed the spherical shape of 65nm. The crystalline structure and size of biologically and chemically synthesized CuO nanoparticles were analyzed by using XRD analysis, a series of diffraction peaks and plane values showing hexagonal phase (JCPDS-80-1916) and 67nm and 65nm in size respectively. Similarly, XRD diffraction peaks are indexed as 29.40(77.6), 36.80(110) 42.10(200)61.90(220) and 1110(222) for biologically synthesized CuO Nps using alga (Bifurcaria bifurcata) extract [22]. The CuO Nps synthesized by the hydrothermal method is indexed as monoclinic phased CuO NPs by comparison with a Joint committee on Powder Diffraction Standards (JCPDS) card files No. 80-1916 [23]. The XRD patterns of all the diffraction peaks are in good agreement with the standard diffraction data for CuO (JCPDS 45-0937), no characteristic peaks were observed for other oxides (such as Cu2O or Cu2O3) and the average crystallite size estimated by the Debye-Scherer equation was about 71 nm [10].
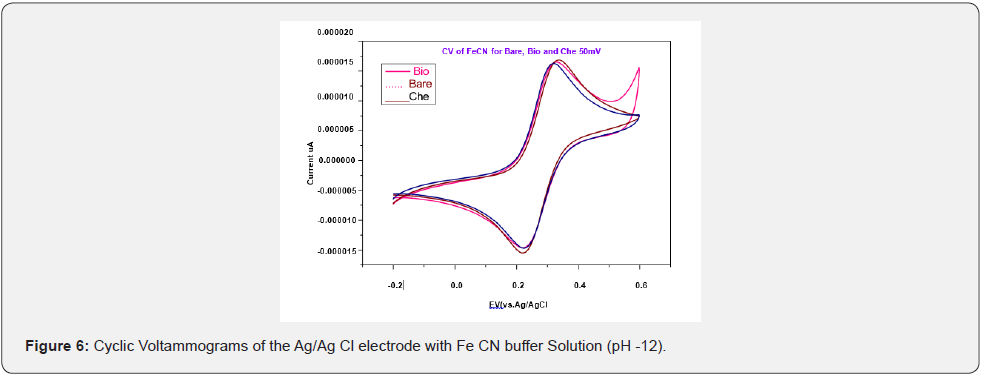
Biologically and chemically synthesized CuO Nps were used for the detection of melatonin hormone in serum samples to study their chemical sensing properties. The electrical responses of both chemically and biologically synthesized CuO Nps were tested using CV (Cyclic Voltammograms) and compared to the bare electrode with Fe CN buffer solution (Figure 6). The electrical response of biologically and chemically synthesized CuO Nps were measured CV in the presence of melatonin hormone (Figure 7). SWV (Square wave value) of Ag/Ag Cl electrode of CuO Nps with melatonin hormone in NaOH buffer solution to the analysis of different scan rates is presented in (Figure 8a).

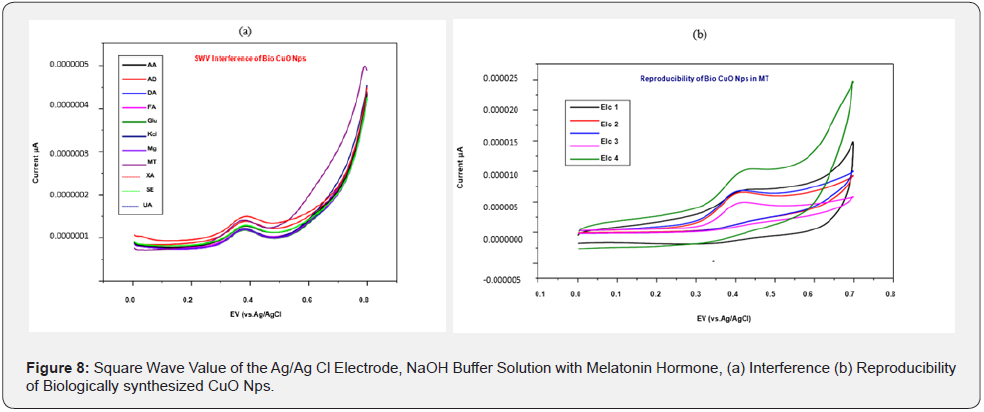
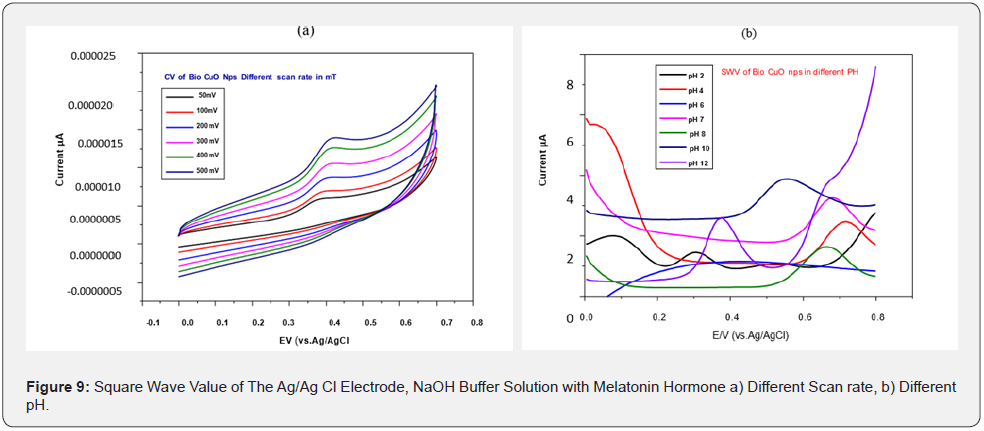
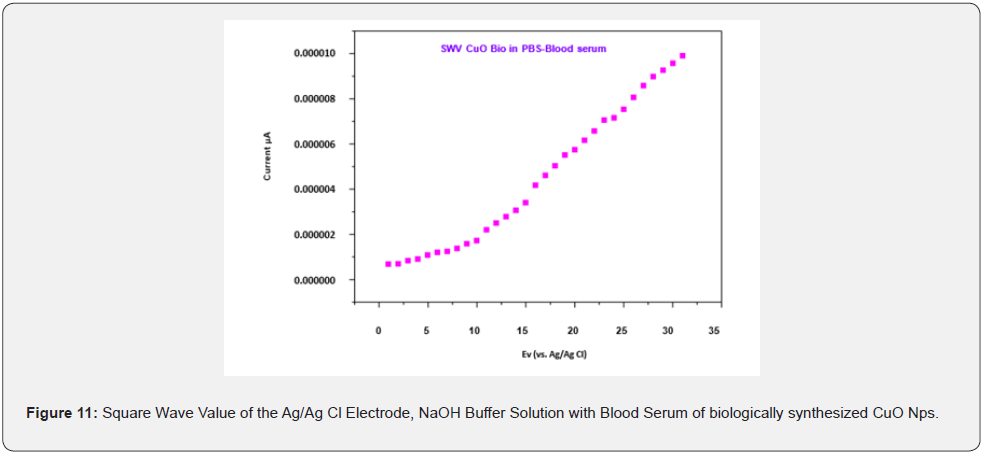
The different pH of the buffer solution is kept constant because increasing the pH affects the efficiency of the electrochemical experiment which may be due to an increase in ion carriers. It is observed at pH 12, the biologically synthesized CuO Nps are highly potential than chemically synthesized CuO Nps (Figure 8b). Square wave value of Ag/Ag Cl electrode of CuO Nps with melatonin hormone in NaOH buffer solution is analyzed for the sensing capacity of CuO Nps using different chemicals. Biologically synthesized CuO Nps shows Melatonin sensing capacity of higher than chemically synthesized CuO Nps (Figure 9a) and its reproducibility (Figure 9b). Square wave value of Ag/ Ag Cl electrode of CuO Nps with melatonin Hormone in NaOH buffer solution and biologically synthesized CuO Nps stability is 0.000005 to .000030 current u A (Figure 10). Square wave value of the blood sample, sensing of melatonin hormone by using biologically synthesized CuO Nps with NaOH buffer solution at pH 12 is 0.000000 to 0.000010 current uA (Figure 11).

The chemical sensing performance of biologically and chemically synthesized CuO Nps was investigated by various chromatographic and spectroscopic techniques. Melatonin hormones were detected by electrochemical properties at pH 12. This result, clearly explains that the biologically synthesized CuO Nps are highly potential than chemically synthesized CuO Nps in sensing melatonin hormone. A similar study was reported on the electrochemical performance of the CuO- graphene modified glassy carbon electrode for glucose sensing by cyclic voltammetry (CV) and amperometry I-t Curve [24,25]. The CuO graphene-modified electrode shows good electrocatalytic activity towards the oxidation of glucose in 0.1M NaOH solution which is highlighted by a simple preparation process for a highly sensitive and selective DA sensor along with an in-depth characterization.
Plectranthus amboinicus leaf extract was employed for the biosynthesis of ZnO nanoparticles and a novel electrochemical sensor was fabricated based on the ZnO NPs coated glassy carbon electrode (GCE) and also used for the detection of the organic pollutant hydrazine [26]. Different shaped CuO nanoparticles were synthesized using cetyl trimethyl ammonium bromide (CTAB) and sodium dodecyl sulfate (SDS) in a co-precipitation method [27]. The prepared CuO nanoparticles were used for the preparation of modified carbon-paste electrodes (MCPE) for the electrochemical detection of dopamine (DA) at pH 6.0. Also reported that the synthesized CuO Nps have been used to construct nonenzymatic glucose sensors, and sensors exhibited highly enhanced electrocatalysis towards glucose oxidation compared with that of a bare graphite electrode [28]. Though the CuO flowers/G electrode can produce a high sensitivity of 709.52A/mM with the initial injection of 0.5mM glucose and a low detection limit of 4mM, due to its slow electron transfer. The electrocatalytic reduction of hydrogen peroxide in an alkaline medium at a carbon ionic liquid conductor changed with copper oxide nanoparticles [29].
The combination of the good conductivity of the ionic liquid and the high catalytic activity of the nanoparticles resulted in an electrode with attractive properties for the determination of hydrogen peroxide. The linear range for the determination of hydrogen peroxide is from 1.0 μm to 2.5 mm, the detection limit is 0.5 μm high stability, sensitivity, selectivity and reproducibility. The fluorescent hydrogen peroxide was developed based on the peroxidase activity of cupric oxide nanoparticles. Cu Nps completely catalyzed the hydrogen peroxide into hydroxyl radicals [30]. The terephthalic acid was oxidized by hydroxyl radical to form a highly fluorescent product.
The linear arrangement of hydrogen peroxide is estimated to be 5.0 ×10-6 to 2.0 ×10-4 M with a detection limit of 3.4 × 10-7M and sensitive assays of glucose and lactate with detection limits of 10×10-6 and 4.5×10-8M. Also reported that the chemiluminescent cholesterol sensor was constructed based upon the peroxidase with good selectivity and enhanced sensitivity like the activity of cupric oxide nanoparticles. Cupric oxide nanoparticles catalyze the oxidation of luminal hydrogen peroxide, which was produced by the reaction of cholesterol oxidase [31]. Therefore, the oxidation of cholesterol into the chemiluminescence of luminal by combining these two reactions. The CL intensity was proportional to the concentration of cholesterol over the range of 0.625 – 12.5 mM and a detection limit was 0.17Mm Conclusion: Biologically synthesized CuO Nps are highly potential than chemically synthesized CuO Nps in sensing melatonin hormone.
Conclusion
The concluded that biologically and chemically synthesized copper oxide nanoparticles are used as biosensors for melatonin hormone.



I admire this article for the well-researched content and excellent wording. I got so involved in this material that I couldn’t stop reading. I am impressed with your work and skill. Thank you so much. Home Broadband Connection At Lowest Price
ReplyDelete