Gastroenterology & Hepatology - Juniper Publishers
Abstract
Colorectal cancer (CRC) is a kind of incurable cancer with high morbidity and mortality. In recent years, mounting evidence has revealed that inflammatory bowel disease (IBD) was correlated with colitis-associated cancer (CAC). Signal transduction is an effective way for cells to respond to external stimuli and eventually trigger specific biological effects. The occurrence, growth and metastasis of cancer are usually associated with disorders of signaling pathways. Although modern research can elucidate the pathogenesis of CAC and provide enhanced screening strategies, the prevalence of CAC is still rising. Studies have shown that several cells signaling pathways in CAC are dysfunctional, leading to the occurrence of malignant phenotypes. Therefore, analyzing the signaling pathways involved in CAC metastasis is necessary to elucidate the potential mechanisms of CAC progression and drug treatment. Our understanding of CAC-related signaling pathways is largely due to a mouse model that faithfully reproduces human CAC. In particular, chemical models can quickly and effectively analyze the molecular mechanism of CAC without increasing time-intensive genetic hybridization. Therefore, this article summarizes the following three chemical-induced CAC animal models, azoxymethane/dextran sodium sulfate (AOM/DSS), 1, 2-dimethylhydrazine (DMH)/DSS and 2, 4, 6-trinitrobenzene sulfonic acid (TNBS)/AOM. Among them, DSS and TNBS were used as inflammatory agents to simulate the pathogenesis of IBD inflammatory models, while AOM and DMH were used to producing carcinogenic methylated DNA, combined with inflammatory agents, could better induce IBD to CAC in animals.
Keywords: Colitis-associated cancer; Inflammatory bowel disease; AOM/DSS; DMH/DSS; TNBS/AOM
Abbreviations: CRC: Colorectal Cancer; IBD: Inflammatory Bowel Disease; CAC: Colitis-Associated Cancer; AOM: Azoxymethane; DSS: Dextran Sodium Sulfate; DMH: 1, 2-Dimethylhydrazine; TNBS: 2, 4, 6-Trinitrobenzene Sulfonic Acid; ACF: Abnormal Crypt Foci; UC: Ulcerative Enteritis; CD: Crohn’s Disease; MAM: Methylazomethanol; ROS: Reactive Oxygen Species; MDSC: Myeloid-Derived Suppressor Cells; DC: Dendritic Cells; Th: T Lymphocytes; MyD88: Myeloid Differentiation Factor 88; CPB: CREB Binding Protein; GR: Glutathione Reductase; HO-1: Heme Oxygenase-1; TR: Thioredoxin Reductase; PEITC: Phenethyl Isothiocyanate; GSK3β: Glycogen Synthase Kinase 3β; CK1α: Casein Kinase 1α; DVL: Disheveled; TCF: T Cell-Specific Factor; LEF: Lymphoid Enhancer Binding Factor; MDF: Mucin-Depleted Foci; JAK2: Janus Kinase 2; STAT3: Signal Transducer and Activator of Transcription 3
Introduction
Colorectal cancer (CRC), as one of the common malignancies with high morbidity and mortality [1,2], usually occurs at the junction of the rectum and sigmoid colon. It is the result of healthy colonic epithelial cells transforming into cancer [3]. This process is called ‘adenoma-carcinoma sequence’, which is developed through a series of orderly events. The initial step is the transformation of normal colonic epithelium to abnormal crypt foci (ACF). ACF progresses to CRC within 10-15 years [4,5]. Accumulating evidence has shown that its occurrence and development were related to many factors, such as environment, eating habits, congenital heredity, personal physique and so on [6]. Over the past few decades, CRC, including colitis-associated cancer (CAC) has posed a serious threat to people’s health and life, which develops from long-term colitis in inflammatory bowel disease (IBD) patients [7] .
IBD, one of the key influential factors resulting in CAC, is a chronic and recurrent gastrointestinal inflammatory disease that can affect the body’s immune mechanism [8]. Generally speaking, the occurrence of IBD in humans may be related to environmental factors [9]. but in the current research hotspots, diet is also considered to be the cause of IBD to a certain extent [10]. especially the intake of allergenic substances in food [11]. Clinically, IBD is usually divided into two kinds of disease, ulcerative enteritis (UC) and Crohn’s disease (CD) [12,13]. Studies have found that long-term IBD may not only lead to the occurrence and development of CRC, but also increase the risk of prostate cancer [14]. gastric cancer and other cancers [15]. Morever, the risk of colorectal cancer is increased twofold in CD and in UC [16] while about 8% of UC patients develop CAC within 20 years and 18% within 30 years [17].
The rodent carcinogen-induced model recalls a long tradition but still retains its usefulness for some applications. They provide a platform for dietary research and insight into the pathways of food-borne carcinogens and colitis-related carcinogenic effects. Since the development of carcinogen-induced rodent models for CAC more than 80 years ago, these animal models have played a huge role in chemoprevention research and assessment of colitisrelated carcinogenic mechanisms [18]. In 1915, scientists proved the carcinogenic properties of coal tar by repeatedly applying coal tar to rabbit ears [19].
At about the same time, the first researchers studied the occurrence of colon cancer by applying chemical or radioactive substances [20]. In the 1960s, heme and its metabolite methylazooxymethanol have been shown to induce rodent cancer [21,22]. In the following years, the chemically more stable substance AOM and its precursor DMH were widely used to induce colon cancer in mice and rats. Both of the two compounds are metabolized to methylazoformaldehyde, which can alkylate DNA bases guanine and thymine [23]. After two-phase reaction treatment, it is secreted into the bile, and its carcinogenic effect on intestinal mucosa exceeds its carcinogenic effect [24].
The most typical AOM/DSS model needs to be combined with a single injection of AOM, a carcinogen that is metabolized in the liver to the active agent methylazomethanol (MAM), and then use inflammatory damage agent DSS [25]. The pathological manifestations of the AOM/DSS model were severe colitis, accompanied by weight loss and bloody diarrhea, and followed by multiple colon tumors. Another carcinogen DMH undergoes a series of metabolic reactions and eventually reaches the colon, producing the ultimate carcinogen and reactive oxygen species (ROS), which further alkylate DNA and cause colon cancer [26]. TNBS, a classical skin contact ant serves as a happen, which could induce a specifically inflammation model in the gastro-intestinal tract [27].
It was reported that the exact location of tumor in colon length depends on the mouse strain and background [28]. The carcinogenic process of the system has a pathological progression from normal intestinal crypts to crypt fission to lesions with abnormal crypts and eventually to microadenomas [29,30]. These steps outline the sequence of CAC formation in humans from inflammation to developmental abnormalities and to cancer.
In recent years, AOM/DSS [31-33] DMH/DSS [34,35] and TNBS/AOM [36,37] are three main methods to establish CAC animal models, as these three chemical pathways could better simulate the process of IBD to CAC in vivo. Therefore, this paper summarized the mechanism of these three modeling methods which can reflect IBD and CAC as follows.
AOM/DSS
Chemical properties
AOM is a class of chemical compound with the molecular formula of C2H6N2O (molecular weight: 74.08g/mol) and strong oxidative activity. DSS is a kind of polyanion derivative of dextran with the chemical formula of (C6H7Na3O14S3) n), which could be used for modeling CAC, separating lipoproteins and improving nucleic acid hybridization rate.
Modeling method
AOM/DSS model is the most widely used and reproducible method to induce CAC. Mice were intraperitoneally injected with AOM (7mg/kg or 12.5mg/kg) [38]. After 5 days, mice received 3 cycles of drinking 1%-3% DSS solution for 5 days and water for 14 days [39]. At the end of the experiment, the mice showed obvious intestinal polyps with intestinal mucosal injury.
Mechanism
As shown in figure 1, AOM reaches the liver through blood circulation. Under the action of cytochrome P4502E1 [40,41]. it is hydroxylated to form MAM [42]. which can spontaneously decompose into formaldehyde and highly active alkylate after entering the intestine [43]. In addition, some scholars have proposed that MAM may be metabolized through another pathway, that is, the metabolic reaction involving tissue-specific cytoplasmic enzyme ADH in the intestine.
ADH generates reactive methylases, alkylases, or such reactive methylases, which can promote DNA alkylation [44,45], and subsequently induce DNA to produce O6-methylguanine adduct [46], resulting in the conversion of G to A in the DNA base sequence. MAM through the liver, and the product ADH produced by intestinal metabolism of MAM leads to changes in DNA base sequence. And after free drinking of DSS, the balance of T lymphocytes in animals is destroyed, promoting the inflammatory response. Combined use of inflammatory and carcinogenic agents alters the genome sequence of cancer, eventually leading to tumorigenesis. AOM/ DSS is one of the most commonly used chemical reagents to induce CAC in rodents [47] which can better simulate the pathological process of the body from normal intestinal epithelial mucosa to abnormal crypt lesions [48] adenoma and colorectal cancer [49].
Among them, DSS reagent has the characteristics of convenient use and high efficiency [50], as a chemical reagent for inflammatory stimulation, it continuously stimulates the mucosa of intestinal epithelial cells during free drinking, causing repeated inflammation in model animals. In the DSS model, sulfated polysaccharides do not directly induce intestinal inflammation, but act as a direct chemical toxin of colonic epithelium, leading to epithelial cell damage. The mechanism is that oral administration of DSS leads to the destruction of the intestinal epithelial monolayer lining, the entry of intraluminal bacteria and associated antigens into the mucosa, and the transmission of pro-inflammatory intestinal contents to the lower tissues [51-53].
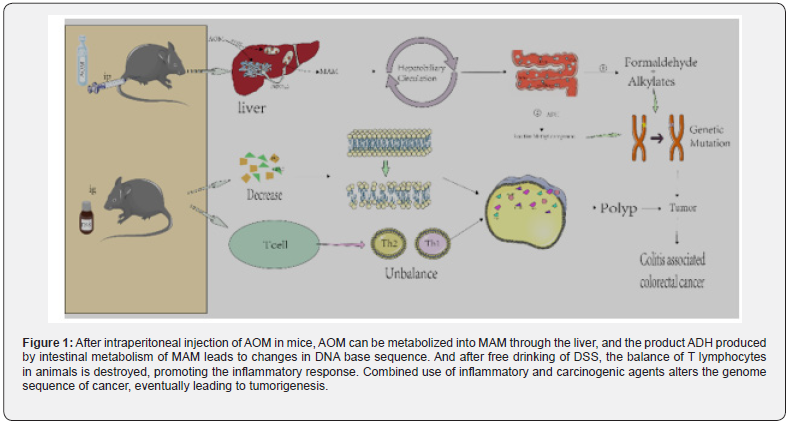
It is generally believed that in chronic inflammation, the immune system is characterized by intensive infiltration of innate immune cells (such as macrophages, neutrophils, myeloidderived suppressor cells (MDSC), dendritic cells (DC) and NK cells and adaptive immune cells (such as T and B lymphocytes), further suggesting that the changes in the intestinal inflammatory microenvironment promote tumor formation [29] In the internal environment of the body, helper T lymphocytes (Th) play an important role in the immune response. The dynamic balance of Th1 and Th2 is one of the necessary conditions to maintain the normal physiological function of the body [54]. Therefore, the increase or decrease of Th1 or Th2 could cause immune response [55] eventually resulting in the occurrence of inflammation [56,57].
After oral administration of DSS in mice, the dynamic balance between Th1 and Th2 was broken, which promoted the expression of inflammatory factors such as TNF-α, IL-1β, IL-10 and IFN-γ, and so on [58,59]. In AOM/DSS model animals, the continuous production of pro-inflammatory cytokines leads to mutations in proto-oncogenes and tumor suppressor genes APC, K-RAS and p53, as well as genomic sequence variations, generating tumorigenesis, progression and metastasis [60]. Myeloid differentiation factor 88 (MyD88), a molecule essential for TLR intracellular signaling, appears to have a protective effect against AOM/DSS-induced inflammation-associated CRC in mice [61]. Yassin M proposed that AOM/DSS-induced inflammation led to an increase in the total number of CD3+, TCRγδ and TCRαβ cells, including a significant increase in CD4+TCRαβ cells, but only moderate changes in CD8αβ TCRαβ and CD8αβ TCRαβ/TCRγδ cells. This suggests that CD4+T cell recruitment plays a key role in regulating various parts of the mucosal immune response during acute and chronic DSS-induced colitis [62].
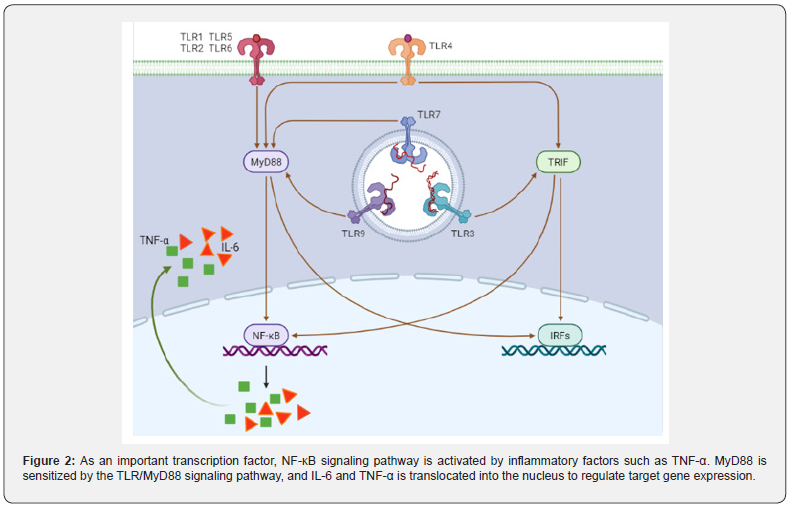
The NF-Κb signaling pathway, a central mediator between inflammation and cancer, promoting CAC development: It is well known that TNF-α can directly activate NF-κB signaling pathway and induce inflammatory response. Furthermore, MyD88 signaling molecules can also activate NF-κB-induced kinase and indirectly activate NF-κB signaling pathway through TLR/MyD88 signaling pathway [63,64] (Figure 2). Xie et al. [65] synthesized a MyD88 inhibitor TJ-M2010-5, which can bind to the TLR domain of MyD88, inhibit the production of its homodimer, and ultimately inhibit the TLR/MyD88 signaling pathway. TJM2010- 5 can significantly reduce the incidence of AOM/DSSinduced colitis, shorten colon length, reduce tumorigenesis, and inhibit the production of inflammatory factors. Moreover, when the TLR4-deficient mice and the wild-type mice were treated with AOM/DSS at the same time, it was observed that the TLR4- deficient mice had a lighter degree of colon cancer than the wild type mice [66]. After the MyD88-/- mice were injected with AOM, colonoscopy showed no tumor formation in the intestine, and the mRNA expression of pro-inflammatory cytokines TNF-α and IL12P40 was lower than that of the model group [67]. On the one hand, the production of TNF-α can activate the NF-κB signaling pathway, on the other hand, when the NF-κB signaling pathway is activated, it can also promote the transcription and translation of TNF-α. El-Daly et al. [68]. showed that in AOM/DSS-treated mice, the positive staining of p65 in the nucleus of the vagus nerve crypt of colorectal epithelial cells gradually increased with the increase of DSS administration cycle. In addition, AOM/DSS also induced the high expression of TLR4 and p65 proteins upstream of NF-κB signaling pathway. Furthermore, Song et al. [69]. found that the expression level of pro-inflammatory enzyme COX-2, which is mainly regulated by NF-κB signaling pathway, was significantly higher than that of the control, and the expression levels of the other two target genes TNF-α and IL-6 also tended to increase [70].
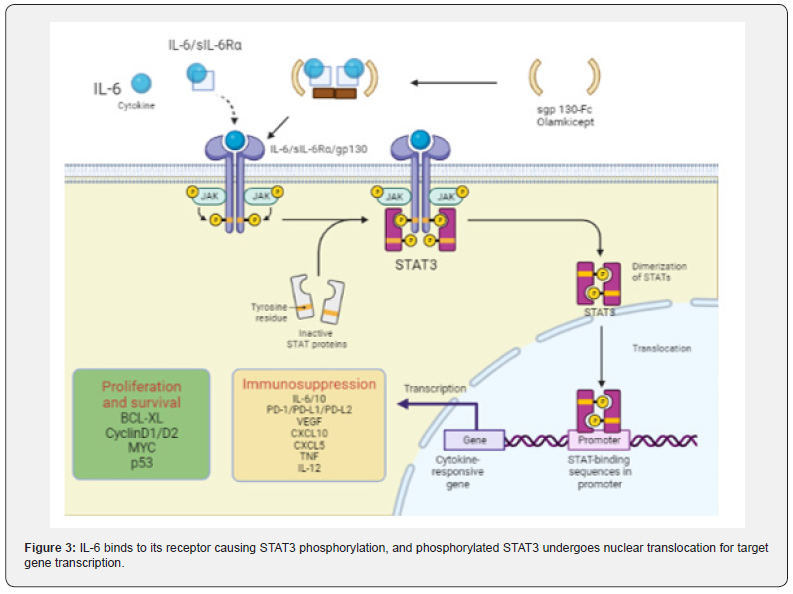
The IL-6/STAT3 signaling pathway is enhanced and accelerates carcinogenesis in CAC: As an inflammatory cytokine, IL-6 binds to soluble IL-6 receptors to form an IL-6/ sIL-6R complex [71,72]. which activates gp130 expression on the cell membrane, leading to signal transducer and activator of transcription 3 (STAT3) phosphorylation. STAT3 enters the nucleus in the form of dimers and activates the STAT3 signaling pathway (Figure 3) [73]. Studies have shown that p-STAT3 staining in the colorectal region of AOM/DSS-treated mice was deepened, and the deposition of brown granules in cytoplasm and nucleus was enhanced with the increase of the administration cycle [74]. MiR-18a is a transcription product in the STAT3 signaling pathway, in AOM/DSS-treated mice, it was found that the expression of MiR- 18a increased significantly with the extension of DSS treatment cycle [75]. but the expression of PIAS3 (an inhibitor of STAT family activation) decreased. In vitro studies have shown that the expression level of MiR-18a is negatively correlated with PIAS3. MiR-18a can negatively regulate PIAS3 [76]. while overexpression of PIAS3 leads to a decrease in IL6-activated STAT3 activity. Ma et al. reported that the proliferation and survival of precancerous intestinal epithelial cells (IECs) were regulated by IL-6/STAT3 signaling pathway, and CAC may be related to this signaling pathway [77].
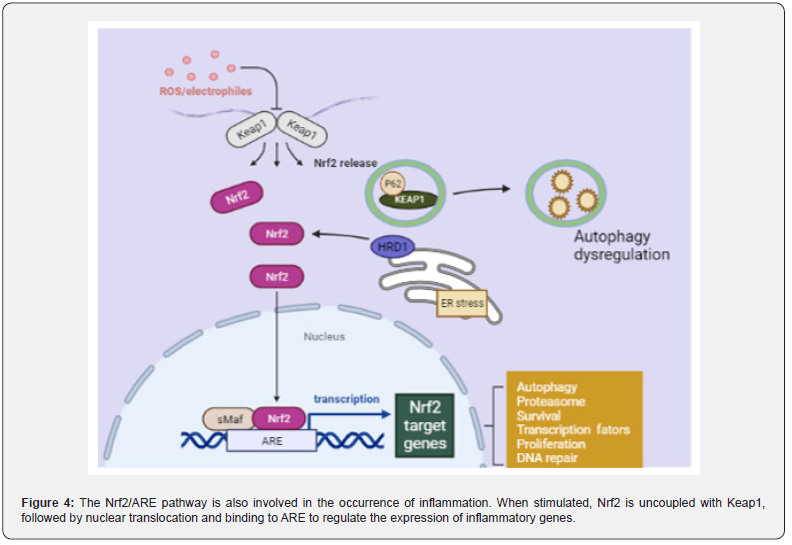
Nrf2/ARE signaling pathway plays an inflammatory role in CAC: In addition, the occurrence of inflammation is also related to the activation of the antioxidant Nrf2/ARE signaling pathway. When the Nrf2/ARE signaling pathway is closed, Keap1 in the cytoplasm specifically binds to the N-terminus of Nrf2, thereby maintaining the normal physiological function of the body. However, when cells receive external stimuli, the Nrf2/ARE signaling pathway is immediately activated, Nrf2 is uncoupled with Keap1, and then enters to the nucleus and binds to the small may protein to form a heterodimer. This process requires the participation of transcription cofactor CREB binding protein (CPB).
Heterodimers specifically recognize ARE and initiate transcription and translation of target genes, resulting in inhibition of the Nrf2/ARE signaling pathway (Figure 4). When the antioxidant process in the body is initiated, the expression of antioxidant proteins is significantly increased to protect cells from damage [78,79]. Wang et al. [80]. found that the positive expression of Nrf2 and the expression of glutathione reductase (GR), heme oxygenase-1 (HO-1) and thioredoxin reductase (TR) were significantly degraded in AOM/DSS treated mice. The researchers [81]. fed mice with 0.05% phenethyl isothiocyanate (PEITC) before and after AOM/DSS administration, found that the incidence of intestinal adenomas in mice decreased by 37.5% and 40%, the number of polyps reduced to 0.375 and 0.6, and the average size decreased to 1.5 and 2.6 mm, respectively. It can be inferred that PEITC can improve the development of intestinal tumors, as an activator of Nrf2, PEITC can activate Nrf2/ARE signaling pathway. In addition, some scholars performed Nrf2 gene knockout in wild AOM/DSS model mice, early model mice and 9-week model mice [82]. It was proved that Nrf2-/- mice showed the same phenotype as wild-type mice, but the incidence of tumors and the proportion of adenocarcinoma were higher.
DMH/DSS
Chemical properties
DMH is a class of compound whose molecular formula is C5H8N2O2 (molecular weight: 128.13g/mol) and provides raw materials for the synthesis of proteins in vivo.
Modeling method
Scientists [83] injected DMH (40mg/kg) into FMH rats three times in the first week, and then gave 1% DSS solution to drink freely for one week, repeating three cycles. DMH is a precursor of the carcinogen AOM, which is not carcinogenic in itself, but can be carcinogenic by oxidative dealkylation to form AOM [84]. The study confirmed that DMH-treated rats had traditional tubular or villous adenomas and serrated adenomas [85] in their colorectal regions, and it is generally believed that most colorectal cancers progress from adenomas.
Mechanism
As a proto-oncogene, DMH can be decomposed into azomethane and methylazomethanol under the catalysis of liver enzyme system. The latter substance flows to the colon tissue through the hepatobiliary circulation and is easily decomposed into a carcinogen methyl diazo ion [86] in the circulation. Methyl diazo ions can methylate DNA, RNA and proteins in normal intestinal epithelial cells. When they reach the colorectal site, they can produce activated carbon ions, leading to oxidative stress, over-expression of inducible nitric oxide synthase (i-NOS) and COX-2 [87], which in turn causes colorectal cancer.
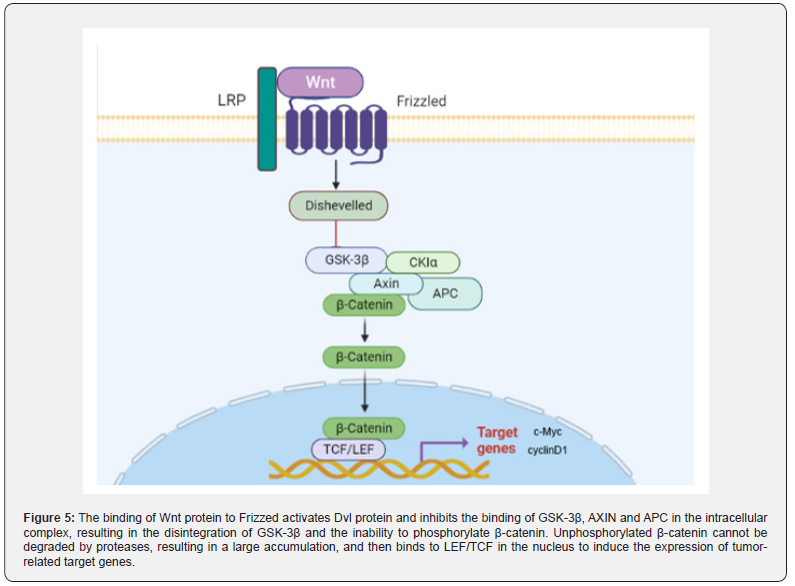
Up-regulated Wnt/ β -catenin signaling pathway promotes CAC progression: Wnt/β-catenin signaling pathway is a conserved signaling axis involved in a variety of physiological processes, such as proliferation, differentiation, apoptosis, migration, invasion and tissue homeostasis [88,89]. In the Wnt/β-catenin pathway, the abnormal regulation of the transcription factor β-catenin can lead to the occurrence of early cancer events [90,91]. In the degradation complex of this pathway, glycogen synthase kinase 3β (GSK3β) and casein kinase 1α (CK1α) mediate phosphorylation of β-catenin, promoting its ubiquitination and subsequent proteasome degradation [92]. The β-catenin dependent signaling pathway is triggered by the binding of secreted cysteine-rich glycoprotein ligands Wnts to LRP-5/6 receptors and FZD receptors. In the presence of Wnt ligands, the binding of Wnt ligands on the cell surface to the receptor induces disheveled (DVL), leading to the aggregation of complexes (AXIN, GSK3β, CK1, APC) to the receptor [93]. Subsequently, phosphorylation and inhibition of GSK3β ensured an increase in cytosolic β-catenin concentration. Unphosphorylated β-catenin in the cytosol migrates to the nucleus and accumulates, interacting with T cell-specific factor (TCF)/lymphoid enhancer binding factor (LEF) to trigger Wnt target genes such as c-Myc [94]. and cyclin D1 (95), resulting in up-regulation of TCF/LEF target genes [95].
In the analysis of 11 DMH/DSS-induced adenocarcinoma mice, Tanaka observed markedly enhanced expression of the β-catenin gene in 10 of 11 mice [96]. Furthermore, mutations of β-catenin, Apc and K-ras genes and cytoplasmic β-catenin expression were found in DMH-induced mucin-depleted foci (MDF) [97,98]. MDF is a microscopic dysplasia induced by specific colon carcinogens in the colon of rodents. Most MDF show Wnt pathway activation, while only one subset shows Ctnnb1 gene mutation, encoding β-catenin [99]. It is well known that β-catenin is a key upstream regulator of the Wnt signaling pathway. The activation of Wnt signaling pathway can lead to the increase of β-catenin into the nucleus (Figure 5), which leads to the increase of transcription of tumor-related factors and induces the occurrence of tumors in vivo.
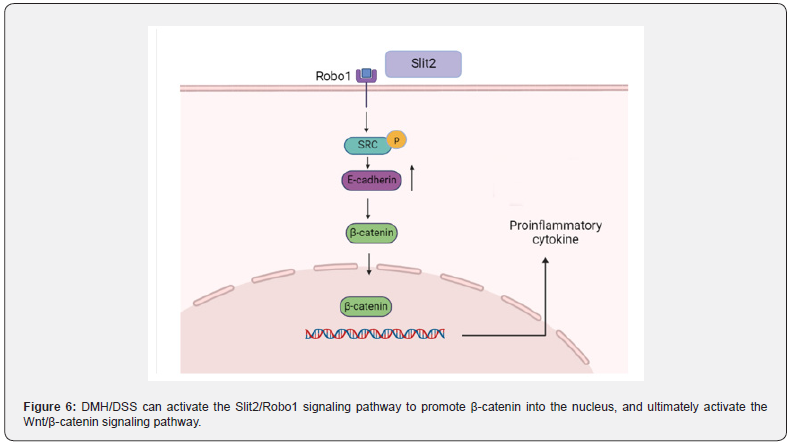
The Slit2/Robo1 signaling pathway induces the Wnt/β -catenin signaling pathway dysregulation to promote CAC progression: Slit/Robo signaling pathway includes Slit secreted protein family (Slit1, Slit2, Slit3) and specific receptors (Robo1, Robo2, Robo3, Robo4). The Slit2/Robo1 signaling pathway was initially found to be involved in the development of the central nervous system. In recent years, more and more studies have reported that it can induce tumorigenesis and participate in the proliferation and migration of tumor cells [100]. The activation of Slit2/Robo1 signaling pathway can be caused by ectopic expression of Slit2 protein (Figure 6). The Slit2 and Robo1 genes on chromosomes are located respectively at 4P15.2 and 3P12.3, Robo1 is a specific receptor of Slit2 [101]. The extracellular IgG region of Robo1 is an important position for binding to Slit2, and the intracellular region is a site for interaction with related signal molecules, which is involved in activating the downstream of Slit2/Robo1 signaling pathway and promoting the occurrence and development of tumors [102].
DMH/DSS can not only directly activate the Wnt signaling pathway, but also indirectly activate the Wnt signaling pathway by activating the Slit2/Robo1 signaling pathway Qian et al. [103]. found that the expression of Slit2 and Robo1 in DMH/DSS mice gradually increased with time. Morever, seven pairs of CRC tissues and non-cancerous colon tissues were randomly selected from patients with N0 CRC (no metastasis). The expression profiles of Slit2 and Robo1 were evaluated by immunohistochemical staining. It can be seen that the expression of Slit2 and Robo1 in CRC tissues is significantly higher than that in non-cancerous colon tissues. As long as the Slit2/Robo1 signaling pathway is activated, it can promote the activation of Src phosphorylation, resulting in increased expression of E-cadherin, thereby activating the Wnt/β-catenin signaling pathway, promoting β-catenin into the nucleus, leading to an increased expression of tumor-associated proteins.
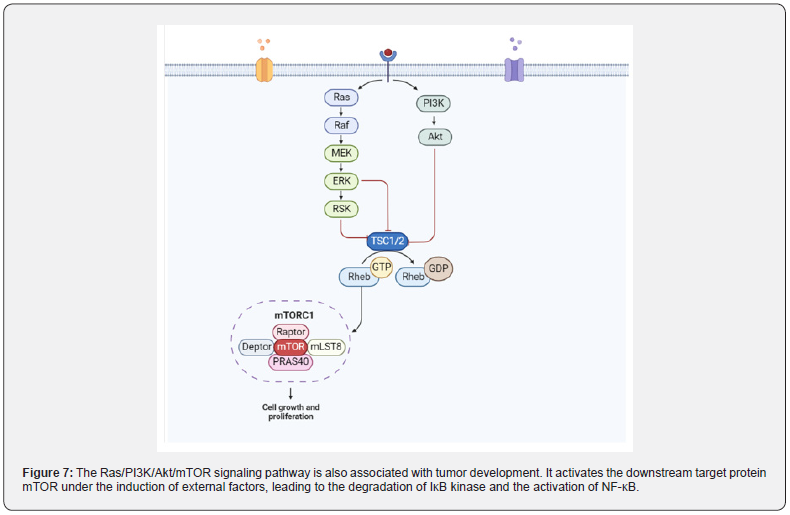
The Ras/PI3K/Akt/M TOR signaling pathway promotes the development of CAC by activating NF-κB protein: The carcinogenic activation of Ras protein caused by missense mutations is a well-known marker of tumor cell proliferation. Balb/c mice treated with DMH/DSS resulted in a significant increase in Ras protein levels. Activated Ras protein targets MEK to trigger ERK phosphorylation and nuclear translocation to express genes involved in cell proliferation. The levels of activated MEK1/2 and phosphorylated-ERK1/2 was significantly increased in DMH/ DSS-treated animals. In addition, Ras oncoprotein also interacts with PI3K and triggers Akt phosphorylation and subsequent activation. DMH/DSS-treated animals showed significantly increased levels of PI3K and phosphorylated Akt. The expression of NF-κB, a downstream effector of Akt, was also significantly increased. The authors believe that the increased expression of NF-κB can be attributed to chronic inflammation followed by circulatory treatment with the inflammatory agent DSS [104]. The mutation of RAS protein is induced by some external factors [105], and mutant RAS protein interacts with PI3K and causes autophosphorylation of PI3K. Type I PI3K is a heterodimer, the corresponding domain of the P85 subunit on the dimer can interact with the linker protein to recruit the corresponding activation receptor for PI3K, thereby converting PI3K into PIP2 and then into PIP3. PIP3 binds to Akt and PDK1 containing the PH domain, leading to the activation of Akt, which in turn regulates the expression of its downstream target protein mTOR. On the other hand, this binding can also activate IK-B, thereby relieving the inhibition of NF-κB, transferring NF-κB from the cytoplasm to the nucleus, and finally regulating the expression of inflammatory factors in the epidermis (Figure 7).
TNBS/AOM
Chemical properties
TNBS is a kind of dangerous chemical compound with a molecular formula of C6H3N3O9S (molecular weight: 293.17). In bioengineering, TNBS can be carried out determining aminoterminal groups, hydrophilic modification reagents, etc.
Modeling method
Xiao et al. [37] injected AOM (10mg/kg) intraperitoneally into C57BL/6 mice, and then 2.5mg TNBS was dissolved in 150μl 50% ethanol and injected into the mice through the rectum. The changes of intestinal tumors in mice were observed under rectal endoscopy. TNBS-induced colitis is very similar to CD, which can induce transmural colitis (Th1-mediated immune response) with severe diarrhea, weight loss, rectal prolapse [106,107], tissue destructive and recurrent [108].
Mechanism
Normally, TNBS was used in combination with anhydrous ethanol. By enema administration, the intestinal mucosa of the model animals was damaged, so that TNBS penetrated the intestinal wall, combined with the related proteins in the intestine, and caused the intestinal epithelial mucosaltenization. Therefore, intestinal cells are more likely to expose target proteins, stimulate immune cells above the intestinal lamina propria [109], and make target proteins more easily recognized by immune cells [110]. resulting in acute necrosis.
TNBS promotes the expression of the same T cytokines as CD [111]. which is an immune inflammatory disease mediated by Th1 [112]. However, the Th1-mediated cycle is short and mainly mediated by Th17, which is characterized by infiltration of CD4+T cells in the intestine [113]. and severe fibrosis of intestinal wall cells in advanced patients [114]. Alrafas Haider [115] treated TNBS-induced colitis with resveratrol and found that the induced inflammatory T cells (Th17 and Th1), cytokines and transcription factors (Foxp3 and TGF-β) were significantly decreased, and the severity of colitis in mice was significantly increased. In general, TNBS-induced colitis also involves Th17-related cytokines [116].
More and more evidence suggests that the growth and proliferation of Th17 cells depend on IL-23. Therefore, some scholars have proposed that the IL-17/IL-23 axis may also be one of the causes of IBD [117,118]. Cubes [119]. found that I-NOS is associated with TNBS-induced colitis and can cause intestinal mucosal damage in model animals, which is similar to some features of DSS-induced colitis. The pathogenesis of CD induced by TNBS is not clear, but the mutation of NOD2 gene was found in C57 mice treated with TNBS, and the mutation of NOD2 gene is related to the pathogenesis of CD. Hence, it is concluded that TNBS can induce CD-related enteritis in model animals [120].
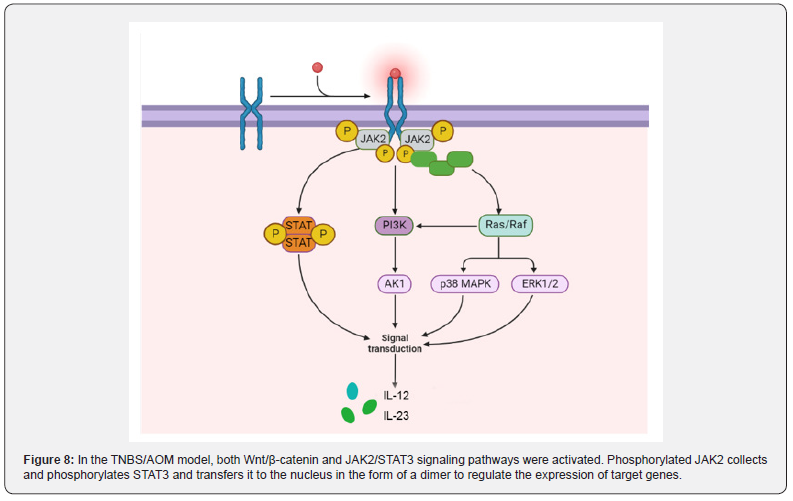
The JAK2/STAT3 signaling pathway plays an oncogenous role in the development and progression of CAC: JAK contains four tyrosine kinases (JAK1, JAK2, JAK3 and TYK2), while STAT contains seven transcription factors (STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B and STAT6). JAK2(Janus kinase 2)/STAT3 pathway is an important signal transduction pathway in the body, which plays a role in many physiological and pathological processes such as immunity, cell proliferation, differentiation, apoptosis and inflammatory response [121]. Under the stimulation of certain cytokines, JAK2 is activated, and then STAT3 is activated, which can transmit extracellular signal into the nucleus and regulate the expression of related inflammatory factors [122]. In the TNBS/ AOM animal model, the activation of Wnt/β-catenin and JAK2/ STAT3 pathways occurred with the development of colitis to adenoma.
He et al. [123]. confirmed that in TNBS-treated mice, Ang II was significantly elevated and the JAK2/STAT3 signaling pathway was activated to induce colitis by stimulating phosphorylation of JAK2 and STAT3. The colitis of TNBS mice treated with JAK2/ STAT3 pathway inhibitor tofacitinib was significantly improved. Lu et al. [124] found that TNBS can not only induce colitis, but also accompanied by visceral allergies. Subsequently, Wan [125] found that the expressions of JAK2, STAT3, p-JAK2 and p-STAT3 was upregulated in TNBS-treated goats. JAK2 is one of the four members of the JAK family located on the cell membrane, specifically binds to some transmembrane receptors and is phosphorylated by internal conformational changes. Phosphorylated JAK2 recruits a large number of STAT3, leading to STAT3 phosphorylation and nuclear translocation in the form of dimers, ultimately mediating the expression of downstream target genes (Figure 8).
The Wnt/β-catenin signaling pathway plays a tumorigenic role in CAC: Zhao et al. [126]. showed that the expression of TCF, β-catenin and Wnt upstream proteins in the TNBS group was significantly higher than that in the blank group, and the expression of tumor-associated proteins c-Myc and cyclinD1 was also significantly enhanced. In the Wnt/β-catenin signaling pathway, Wnt protein binds to the coiled protein (coiled gene) on the cell membrane. After seven trans membranes [127]. the signal is transmitted to the Dvl protein. Subsequently, the activation of Dvl protein inhibited the binding of intracellular complexes GSK-3β, AXIN and APC, resulting in the dissociation of GSK-3β, and then GSK-3β could not phosphorylate β-catenin [128,129]. Unphosphorylated β-catenin cannot be degraded by proteases, resulting in a large accumulation of β-catenin. This process promotes the entry of β-catenin into the nucleus, where it binds to nuclear LEF/TCF and induces the expression of tumor-associated target genes. Therefore, TNBS-induced colitis can complete the process from CD to CAC under the combined action of AOM.
The Other Mechanism of Three Pharmacological Models
Inflammation, endoplasmic reticulum stress and autophagy
It is confirmed that infection and a large number of inflammatory stimuli could cause endoplasmic reticulum stress and activate the UPR signaling pathway, mainly by promoting Ca2+ release from the endoplasmic reticulum and ROS accumulation. For example, pro-inflammatory cytokines IL-1β and IFN-γ can directly down-regulate the expression of Ca2+-ATPase (SERCA2) on the endoplasmic reticulum to depleted ER Ca2+ stores, thereby causing ERS [130].
In addition, ROS, as an important mediator of inflammatory response, can cause disorders of endoplasmic reticulum folding protein-related functions, leading to the accumulation of unfolded/ misfolded proteins in the endoplasmic reticulum and the release of Ca2+ in the endoplasmic reticulum into the cytoplasm. High concentration of CA2+ leads to mitochondrial dysfunction and produces more ROS [131]. Due to the long-term existence of this feedback mechanism in the body, intracellular ROS is continuously generated and accumulated, and abnormally accumulated ROS can aggravate endoplasmic reticulum stress.
Endoplasmic is the main site for protein folding and quality control. Any disturbance that alters endoplasmic reticulum stress homeostasis may lead to the accumulation of misfolded proteins, the activation of unfolded protein response, autophagy, or apoptosis [132] (Figure 9). Zhang et al. [133] . treated mice with AOM/DSS and found that the endoplasmic reticulum lumen was enlarged, with swelling, vacuoles, and a significant reduction in ribosomes. When the endoplasmic reticulum integrity was destroyed, the expression of GRP78, IRE1α, p-ERK, ATF6 and p-eIF-2α related to endoplasmic reticulum stress was significantly enhanced. Similarly, in TNBS -induced rats [134], the expression of endoplasmic reticulum stress-related proteins ATF6, ATF4, CHOP, BIP and XBP-1s was enhanced. Sharma et al.confirmed that the increased expression of p-PERK, p-eIF2α and chop proteins in rats [135], induced by DMH can lead to endoplasmic reticulum stress.
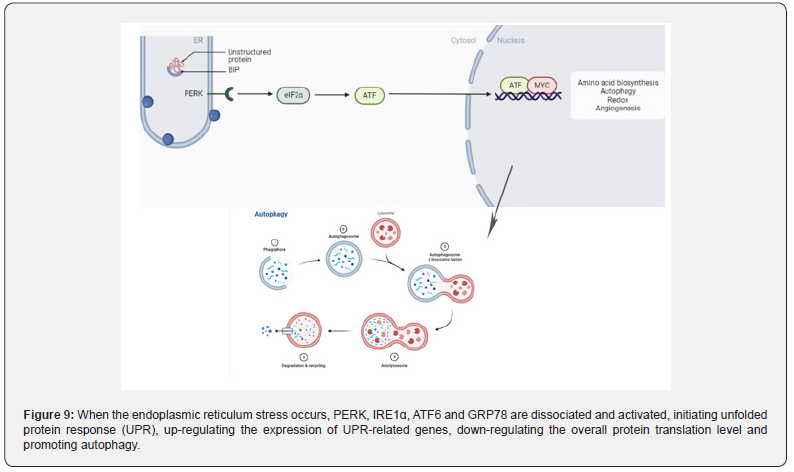
From the mechanism of action, studies have found that IBD-related endoplasmic reticulum stress was associated with autophagy dysfunction [136,137]. Autophagy is a conserved process in eukaryotic evolution through which cytoplasmic materials are degraded in lysosomes [138]. Zhang et al. [133] found that AOM combined with DSS could induce endoplasmic reticulum stress in the intestine of mice and observed a large number of double layers autophagic vesicles in the intestinal epithelial mucosal cells of mice by electron microscopy [138]. The expression of LC3II, an autophagy marker, was also significantly increased. In the DMH/DSS model mice [139]. the expression of P62 and the ratio of LC3II/LC3I were decreased. In TNBS model mice [140], the expression of autophagy related LC3II and autophagy flux were enhanced, and the autophagy ability of intestinal epithelial cells was enhanced.
When endoplasmic reticulum stress occurs in the body, autophagy function is activated [141], which in turn destroys the intestinal mucosal barrier and affects the physiological function of normal intestinal epithelial cells. Subsequently, the diversity of intestinal flora decreased, flora dysfunction [142]. and inflammatory cell infiltration [143] induced IBD, then developed into CAC. AOM/DSS, DMH/DSS and TNBS/AOM can well induce autophagy and endoplasmic reticulum stress during the progression of IBD to CAC.
Intestinal microbial imbalance
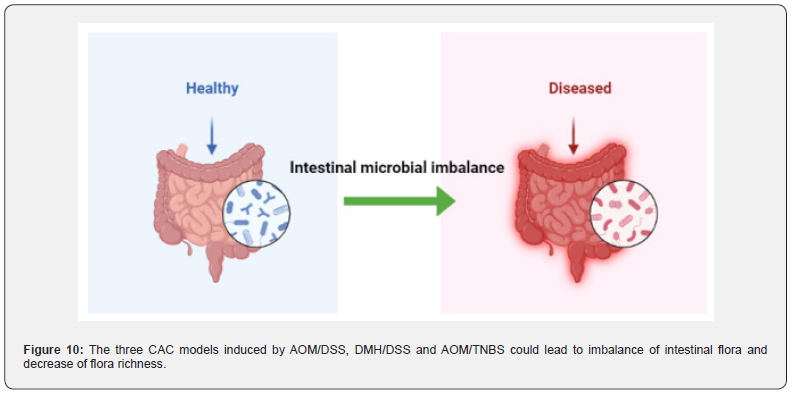
The intestinal epithelium is a monolayer of cells that serves as a physical barrier separating the mucosal immune system from symbiotic and pathogenic microorganisms [144]. Epithelial damage or increased epithelial permeability can destroy this barrier, thereby activating the mucosal immune system. Increased intestinal permeability is observed in patients with Crohn ‘s disease before clinical onset or recurrence, suggesting that barrier dysfunction may trigger disease progression [122,145]. The mechanism of IBD caused by intestinal flora imbalance is mainly divided into the following three aspects: the increased number of pathogenic bacteria, the decreased number of probiotics, and the imbalance of flora to break the normal immune tolerance of the body.
At present, the common human pathogenic bacteria are mostly found in Proteobacteria, and the bacteria belonging to Proteobacteria play an important role in the occurrence and development of IBD, especially Escherichia coli. When the intestinal flora is out of balance, the number of mutual pathogenic bacteria increases (such as adhesive invasive Escherichia coli, etc.). Pathogenic bacteria can destroy the normal internal mechanical barrier and immune barrier through invasion and secretion of toxins, increase the permeability of intestinal mucosa, and create conditions for the shift of flora. On the other hand, transferred bacteria over activate the immune response, causing indirect tissues to be attacked and damaged by the immune system [146]. which further increases the intestinal inflammatory response.
Relevant studies have found that the intestinal mucosal permeability of CD patients is significantly higher than that of normal people, resulting in a large number of bacteria and toxins in the intestine entering the intestinal mucosa and activating immune cells, which will trigger a strong immune response and destroy the mucosa [147]. This may be related to a series of reactions caused by the increase in the number of pathogenic bacteria in CD patients (Figure 9).
There are abundant probiotics in human body, such as Lactobacillus in Firmicutes, Bifidobacterium in Actinobacteria and Bacteroides in Bacteroidetes. When the intestinal flora is imbalanced, the number and richness of Firmicutes and Bacteroidetes in the normal body are reduced, and the number of probiotics such as Lactobacillus and Bifidobacterium is significantly reduced. The above functions are weakened, resulting in impaired intestinal mucosal barrier and proliferation and translocation of pathogenic bacteria. Studies have shown that the bacterial genus in the built-in bacteria can activate Paneth cells to secrete antimicrobial peptides to produce normal antibacterial effects. When the disease causes a significant increase in pathogenic bacteria and a decrease in bacterial genus, the production of antimicrobial peptides is reduced, which further causes excessive immune activation and damage to the intestinal mucosa [148].
Physiologically, there is a symbiotic relationship between intestinal microorganisms and hosts, which depends on a variety of immune mechanisms, such as intestinal mucus secreting immunoglobulin IgA and releasing antimicrobial peptides [149]. In IBD patients, the intestinal microecology is not regulated, and the imbalance of intestinal flora increases the expression and release of inflammatory factors. The normal immune balance of the intestine is broken [150], and the abnormal immune response is activated, resulting in intestinal mucosa and tissue damage.
In AOM/DSS [151] and DMH/DSS [152] treated model animals, the abundance of Firmicutes increased and the abundance of Bacteroidetes decreased, resulting in a decrease in microbial diversity in vivo. In TNBS-induced model animals [153], the intestinal flora composition, species richness and diversity of the model group were significantly different from those of the control group, and the Proteobacteria and Bacteroidetes were significantly increased. After transplantation of fecal microbiota (FMT) in mice, the abundance of these two floras returned to normal levels and microbial diversity increased significantly by observing Shannon curve, PD forest index and CHAO1 index [154] (Figure 10).
Comparison of Three Animal Models (Table 1)
Advantage and disadvantage
AOM/DSS is the most widely used chemical reagent among the three models [155]. It is easy to use, easy to operate, and has a high tumor formation rate that can target tumor formation in animal colorectal regions. At the same time, it has the histological order change of ‘inflammation-dysplasia-carcinoma’. However, there are still some shortcomings in this model. For example, there is a lack of research on cancer stem cells in this model, and the mutations of tumor suppressor genes APC and P53 are rarely seen [156]. CRC has a low tendency of distant metastasis, and the life span of mice is shortened after illness, so it is not suitable for tumor invasion related research [28].
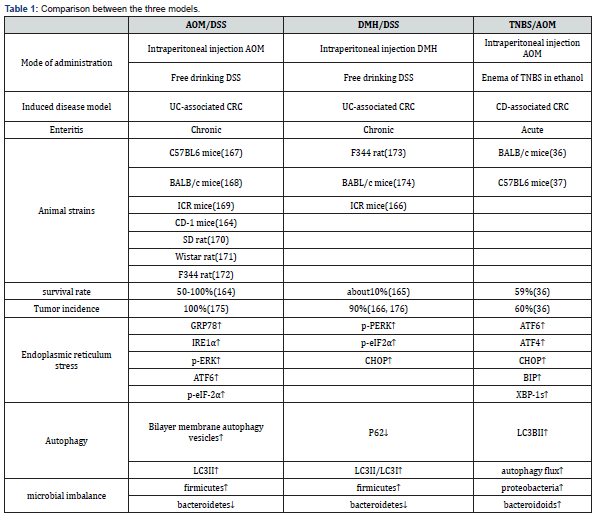
DMH/DSS-induced colon tumors in rodents are very close to human colon cancer in morphology, growth pattern and clinical manifestations [157]. And DMH-induced colorectal adenocarcinoma in mice often invades the submucosa and muscular layer, while AOM-induced colorectal adenocarcinoma does not exhibit such biological and histological properties [158]. However, the main disadvantage of the model using DMH is that multiple injections of DMH and long experimental periods are required to induce colon tumors in experimental animals. Secondly, after DMH is injected into the body, there are many uncontrollable factors in the process of producing AOM by oxidation, and its feasibility is not as high as AOM.
TNBS/AOM, in the construction process, due to TNBS is a semi-antigenic substance, it needs to be combined with ethanol to form a complete antigen, so some researchers use rectal perfusion construction. This model is based on the increased membrane permeability that occurs in IBD, which contributes to the entry of the luminal antigen that immune system cannot fully eliminate, namely haptenization [159]. The main advantages of the model include simple and low-cost protocol and repeatable colonic injury, short duration of the experiment, persistent injury and ulcers with inflammatory cell infiltration. Because of the lack of practice of this technique, the presence of feces in the colon, the anatomical localization of the descending colon, and the volume of the injection rate administered may cause rectal reflux of TNBS, leading to defects in the induction method or increased variability in animal disease.
Pathology
Clinically, IBD patients are also divided into UC and CD. According to Lin et al. [160], UC patients typically present with abdominal pain, increased frequency of diarrhea, and bloody mucopurulent diarrhea. Radiological examination showed obvious edema and hemorrhage of colonic mucosa, diffuse thickening of intestinal wall, occasional cryptitis and deformation of crypt dissection. In CD patients, intestinal wall thickening often occurred in the ileum [161], mucosal thickening and adjacent mesenteric fluid with severe submucosal fibrosis [162]. Saade C et al. [163] reported that approximately 91% of CD patients develop an inflammatory response in the ileum.
In the AOM/DSS-induced mouse UC model, Zhang et al. [133] found that the mice in the model group had pathological symptoms such as diarrhea, vertical hair and anal prolapse, and bloody stools appeared on the 6th day of modeling. Adenoma was diagnosed by histological examination. In Li ‘s experiment, mice in the AOM/ DSS treatment group showed glandular structure disorder and inflammatory cell infiltration. In addition, researchers found that about 80% of mice [164] in the AOM/DSS model group developed adenocarcinoma. In DMH/DSS-induced mice, polyploid adenomatous hyperplasia with malignant transformation of colon, polypoid adenoma [165], thickening of intestinal mucosa, inflammatory cell infiltration [83] bloody stool and rectal prolapse [166].
In TNBS/AOM-treated mice [167], lesions occurred mostly in the distal colon, with colonic crypt abnormalities, accompanied by colorless or polypoidal adenomas, and eventually adenocarcinoma [36]. In addition, some scholars found that AOM/TNBS-induced mice often appear diarrhea [168], bloody stools, colonic mucosal ulcers, submucosal edema and other obvious symptoms of enteritis [37]. Broadly speaking, UC and CD patients will first appear in the body’ s clinical inflammatory response, and then accompanied by abnormal growth of the crypt. However [169], in the three models of AOM/DSS, DMH/DSS and TNBS/AOM, the atypical hyperplasia of intestinal crypt usually appears first, followed by inflammatory cell infiltration and high expression of proinflammatory factors [170].
Conclusion
Various available animal models provide important tools for studying the complex development and pathogenesis of diseases [171]. These models could be used to provide new insights into etiology [172], pathophysiological mechanisms and treatment of human cancers [173]. For example, animal models help us understand the sequential acquisition of genetic and epigenetic changes observed in humans, and the consequent changes in cell behavior and tumor biology [3,159]. Many models are also provided to show the transfer process and the sensitivity to treatment characteristics [174].
Animal studies cannot replace human clinical trials [175], but they must be used in preclinical studies in time so that human diagnosis and treatment-oriented trials can become more concentrated and have a greater chance of success [176]. In fact, these models are not only valuable tools to reveal new mechanisms of CAC pathophysiology [177], but also promising tools to advance our understanding of tumor responses to novel chemoprevention and treatment strategies [178].
Among the three models, AOM/DSS animal model is the most widely used [179,180] the most in-depth studied and the most reductive chemical reagent, which can well simulate the pathogenesis of IBD to CAC [181]. Its operation is simple and convenient [28, 30,182,183] and the success rate and tumor formation rate are higher than the other two modeling methods, and the mechanism of AOM/DSS in vivo is relatively clearer than the other two chemical reagents. Therefore, most of the animal models used for the IBD to CAC process are mainly AOM/DSS [28].
However, the process from IBD to CAC is a complex pathological process induced by multiple factors. Although the model of AOM/DSS-induced IBD to CAC is relatively mature, the effects of diet and environmental factors on the pathogenesis are still not involved [184]. Secondly, the occurrence of cancer is inevitably accompanied by the process of tumor metastasis, but this feature has not been found in AOM/DSS-induced model animals. Thirdly, like any model, the CAC animal model induced by AOM/DSS has its limitations. For example, Kras or p53 mutations are typical in humans, but have not been detected in this mouse model [185]. In contrast, Kras mutations have been observed in CAC rats model using AOM reagent alone [186,187]. In addition, few literatures have compared the pathological features of AOM/DSS, DMH/DSS and TNBS/AOM with clinical related diseases.
If the future technology can combine dietary and environmental factors with the three models of CAC, the clinical tumor metastasis will be modeled in animals, and the clinicopathological features will be compared with the pathological features in the pathogenesis of model animals. It is believed that the pathogenesis of IBD to CAC induced by these three models will be closer to the process of human IBD to CAC, and its pathogenesis will be more comprehensive, providing more reasonable treatment for such patients in the direction of clinical treatment.
To Know more about Advanced Research in Gastroenterology & Hepatology
To Know more about our Juniper Publishers




No comments:
Post a Comment