Oceanography & Fisheries - Juniper Publishers
Abstract
This study investigates the colonization of saltmarshes by the invasive species Magallana gigas in the South West Atlantic region. The research was conducted in the Bahía Blanca and Río Negro estuaries in Argentina. Oyster densities were assessed in different areas of the saltmarshes. The results show varying densities of M. gigas in different locations, with higher densities observed at the edges of vegetated areas in Bahía Blanca and in non-vegetated areas, particularly saltmarsh channels, in the Río Negro estuary. The mechanisms of oyster arrival and their impact on native species and ecosystem functioning are discussed. This research highlights the need for monitoring and managing invasive species to protect saltmarsh ecosystems.
Keywords:Invasive species; Magallana gigas; Saltmarsh ecosystems; Spartina
Introduction
Intertidal marshes, characterized by strong plant zonation and low species diversity but exceptionally high primary and secondary production [1-4], provide valuable ecosystem services such as raw materials, food, coastal protection, erosion control, water purification, support for fisheries, carbon sequestration, and opportunities for tourism, recreation, education, and research [5-11]. Unfortunately, wetland loss worldwide, particularly in the form of marshes [12-14], has been accelerated in the past decade due to factors such as global climate change, sea-level rise, agricultural and industrial development, and sediment supply loss [15]. Although physical factors like wind action, wave energy, and tides contribute significantly to marsh erosion rates [16,17], biological factors interacting with these physical forces also play an important role in geomorphological processes. Autogenic ecosystem engineers and allogenic ecosystem engineers, along with herbivores and their predators, influence primary production and the stability of these environments [4,18-21]. Invasive species further complicate ecological systems by altering the evolutionary pathways of native species, modifying the structure of biological communities, and disrupting habitat complexity [22-25]. Invasive marine invertebrate species pose significant ecological and economic threats to marine ecosystems globally. These species, often unintentionally introduced through ballast water discharge, hull fouling, or aquaculture activities, establish populations in non-native habitats, where they lack natural predators or competitors [26]. Their rapid growth, high reproductive rates, and adaptability enable them to outcompete native species for resources, disrupt marine ecosystems, and cause declines in native biodiversity, impacting fisheries, aquaculture, and coastal communities. Managing and preventing these invasions require effective monitoring, early detection, rapid response strategies, and international cooperation to address this global issue [27].
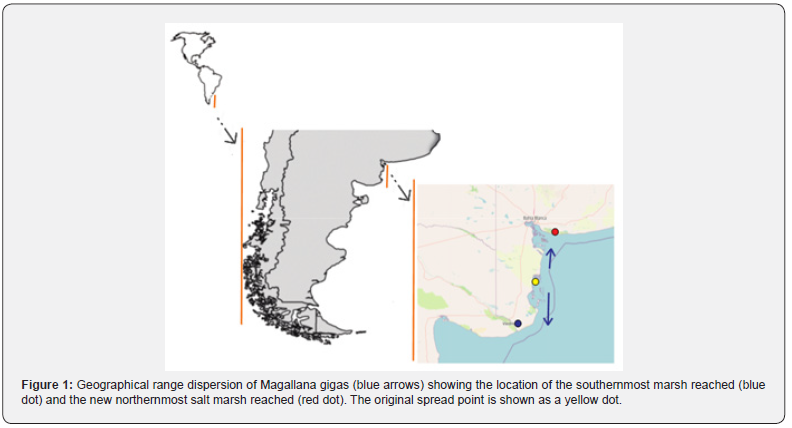
The Pacific oyster, Magallana gigas, intentionally introduced for aquaculture purposes, often becomes invasive, resulting in significant alterations to coastal ecosystems [28]. In Argentina, Magallana gigas was illegally introduced in Anegada Bay (39º 50´ S to 40º 40´S and 62º 10´w) [29,30] around 1982 as part of an oyster culture project [31]. Since its introduction, the first oysters were discovered outside the bay in Los Pocitos in 1987 (40°26’37.08”S. 62°25’20.47”W) [32], and they have since spread both north and south, as reported by Dos Santos et al. [33] who found a few oysters on port docks at the Bahia Blanca estuary (38°52′00″S 62°07′00″W), and by Narvarte & Morsan [34] in the rocky intertidal of El Condor (41° 3’35.84”S 62°50’14.68”w).
(36°29′00″S 56°42′00″W approximately 20 km away from the location). However, [36] later determined that the oysters were another species Crasostrea talonata [37], which negatively impacted S. alterniflora. While the presence of the invasive species Magallana gigas, the Pacific oyster, may have a positive effect on some fish species, as described by [38], since it can be considered an autogenic ecosystem engineer [39], its effect on spartina species remains unknown. Twelve years after the population was established in Bahía Blanca, M. gigas is abundant and has colonized saltmarshes, with apparent significant impacts on ecosystem structure and functionality [38]. However, eighteen years after the population was found in El Condor, it has almost disappeared from the rocks, but it is starting to colonize the saltmarshes in the Rio Negro estuary, a few kilometers away (Molina, pers. obs., this work). In the South West (SW) Atlantic, between southern Brazil (32°S) and northern Patagonia (42°S), extensive saltmarshes dominated by cord grasses such as Spartina densiflora, Spartina alterniflora, and the chickenclaws Sarcocornia perennis are found [40]. These SW saltmarshes, where S. alterniflora is the only or dominant species, are daily flooded by tides (e.g., [38]) and support a high abundance of fishes [38,41].
The aim of this paper is to report the northernmost and southernmost colonization of saltmarshes by Magallana gigas.
While no presence of oysters has been registered in saltmarshes, [35] reported a small population of C. gigas in San Borombon Bay (36°00′S 57°12′W) attached to various structures such as the stems of Spartina alterniflora, plastic bags, wooden sticks, and possibly other mollusk shells, which indicates a population close to an experimental C. gigas farm in Las Toninas.
Materials and Methods
Study Area
The study was conducted in two saltmarshes located in the Bahía Blanca estuary (Villa del Mar, 38° 51’ S, 62° 6’ W) and the Río Negro estuary (40º 58´24” S 62º 48´65” W) in Argentina (Figure 1). These are large macrotidal embayments with tidal amplitudes of up to 4 meters, covering an area of 2300 km2 and 400 km2 respectively, and experiencing semidiurnal tides [42]. In both locations, Spartina alterniflora is the dominant marsh plant species occupying the lower intertidal zone. The middle zone is occupied by Spartina densiflora, while Sarcocornia perennis is restricted to a narrow strip on the higher part of the saltmarsh. During low tide, both the saltmarsh and a wide area of the tidal flat are exposed to air. Sampling was conducted in the summer of 2023 in the lower part of the saltmarsh where S. alterniflora dominates, including vegetated areas and the edge of vegetated areas, as well as in the adjacent tidal channels and tidal flats.
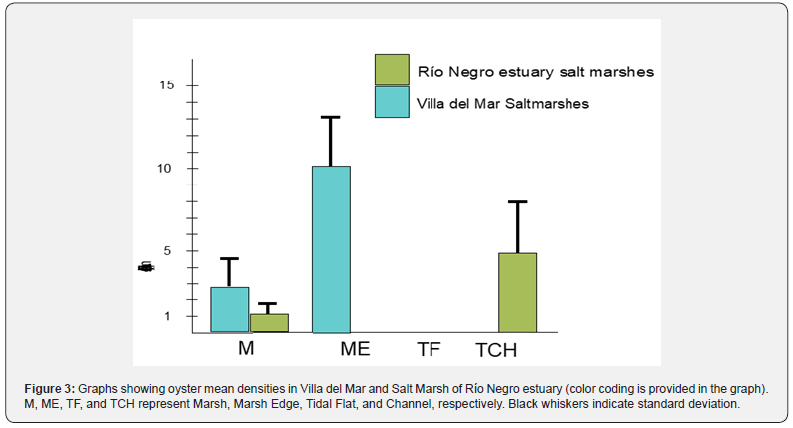
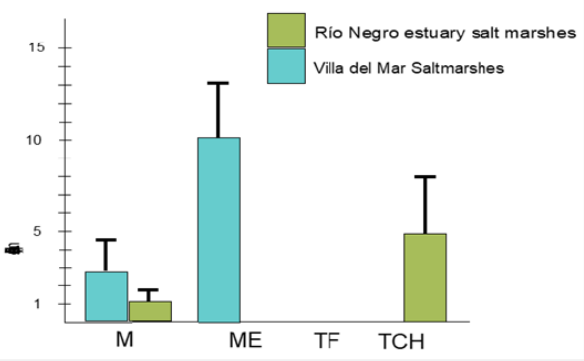
To estimate oyster densities in vegetated areas, channels, and bare sediments on the saltmarshes, we utilized a circular sampler of 0.28 m2 (N=20 per site, Figure 2). Mean densities were calculated and graphed for each site (Figure 3).

Results and Discussion
The density patterns vary between the different sites. In the Bahia Blanca salt marshes, oyster densities are higher at the edges of vegetated areas, while they are lower in the bare sediment areas or tidal flats. On the other hand, in the Rio Negro estuary salt marshes, oyster densities are higher in non-vegetated areas, specifically in the salt marsh channels, compared to the vegetated areas (Figure 3).
In the first location, the substrate consists of a mixture of sand and mud, with extensive bare flats covering most of the intertidal fringe. Despite the limited freshwater input relative to the size of the estuary, there are high densities of Magallana gigas covering the sediment surface, forming reefs. Vegetation is mainly restricted to the upper intertidal zone, with plant densities ranging from 100 to more than 300 ramets per square meter, following a strong seasonal pattern. Interestingly, numerous oysters can be found growing between the plants, resulting in a significant biogeomorphological impact (Molina pers. Obs.).
In the Rio Negro salt marshes, there are no significant differences in sediment characteristics and marsh plant structure compared to the previous location. However, there is a notable difference in the freshwater input to the system, which is considerably higher in this area. As a result, oysters show a preference for salt marsh tidal channels as their preferred habitat. The mechanism by which oysters arrived at both marshes remains unknown, with uncertainty whether it occurred naturally through larval dispersion or through intentional human activities. The lack of larval dispersal models for Non-Indigenous Species (NIS) along the Argentinian coast has led to a significant debate regarding the migration of the Pacific oyster. Additionally, the absence of systematic coastal observations, such as monitoring wind waves, nearshore currents, and other hydrographic features in this specific coastal region, presents a challenge in conducting an unbiased study based on observed parameters. Consequently, there is currently a shortage of peer-reviewed literature on coastal hydrodynamics or littoral processes in the study area due to the limited availability of data [43].
Conclusion
Invasive species have a significant impact on the distribution, abundance, and composition of native species groups. When considering the Pacific oyster (M. gigas) as an autogenic ecosystem engineer, there is an ongoing debate about its effects on biodiversity, with theoretical predictions suggesting both decreases and increases [38], and references therein. This controversy is particularly important in soft-bottom habitats, where M. gigas promotes spatial heterogeneity and complexity by creating reef structures through larval settlement and shell attachment [44].
The overall impact of M. gigas ecosystem engineering is influenced by various factors and interactions. It can facilitate primary producers [43,45], displace native species due to reef structures [46,47], promote further invasions [48,49], and increase sedimentation rates, potentially obstructing coastal areas [46,50]. Additionally, M. gigas can provide new habitat for cryptogenic fish species [38].
The introduction of Magallana gigas, which competes with existing habitat structures such as Spartina, may affect the availability of important refuge and foraging resources for estuarine fishes, while increasing habitat complexity [51,52]. Further studies are necessary to understand the impacts and modifications occurring within communities and their habitats.
This knowledge will enable the prediction of new dynamics and the improvement of management strategies, especially considering that many species inhabiting these habitats may be negatively affected. The loss of stability in saltmarshes, crucial for coastal defense, nurseries, and carbon storage, is a potential consequence that should be taken into account [53,54].
To Know more about Oceanography & Fisheries Open Access Journal
Click here: https://juniperpublishers.com/ofoaj/index.php
To Know more about our Juniper Publishers
Click here: https://juniperpublishers.com/index.php




No comments:
Post a Comment