Pharmacy & Pharmaceutical Sciences - Juniper Publishers
Abstract
Methyl group is one of the most commonly occurring substituents in drugs. More than 50% of drugs with high market value have methyl groups in their structures. Methyl group has a capacity to act as Electron Donating Group and modulating physicochemical and biological properties of drug molecules. These properties include effect on half-life on a drug, selectivity, potency and binding affinity. When introduction of methyl group results in profound increase biological activity of molecules, it is known as Magic Methyl Effect. These enhancements in biological activity are attributed to effect of methyl group on free energy of Desolvation. The present review explores the origin of this magic methyl effect along with some supporting case studies in drug design.
Keywords: Drug design; Magic methyl; Methyl group; Molecules; Drug molecules; Physicochemical; Methylating; Synthetic chemistry; Side effects
Introduction
Methyl group is one of the most common substituents occurring in design of many drugs and research molecules. Methyl group has a capacity to act as electron donating group and is known to modulate physicochemical and biological properties of drug molecules. A survey of Njardarson’s Top 200 Drugs of 2011 showed that more than 67% of small-molecule drugs at that time contained at least one methyl group bound to a carbon atom [1]. Some of the drugs containing methyl group have been listed in the following figure (Figure 1).
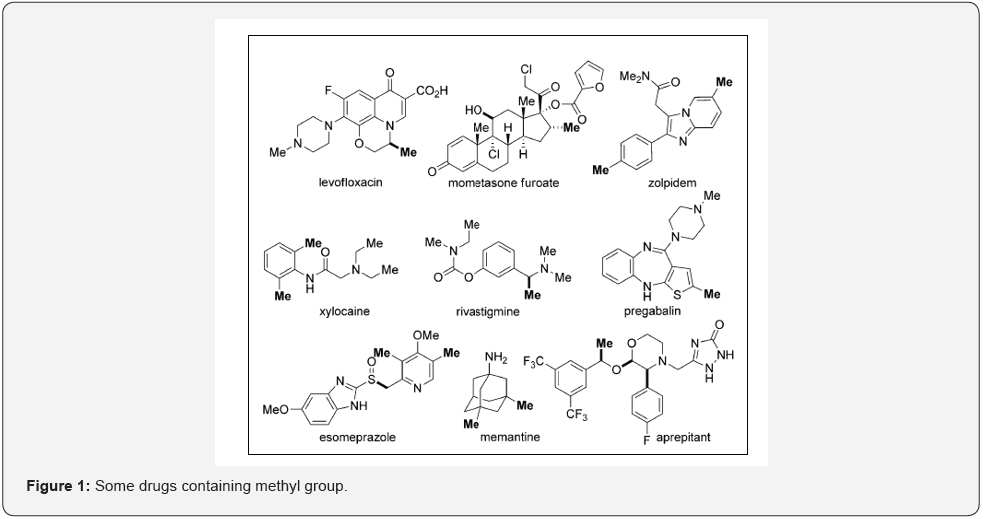
Importance of Methylation
Substituting methyl group in drug design can have various benefits on pharmacokinetics and pharmacodynamics of drug molecules. Some of the effects of methylation are described below.
Alternation in half-life of the drug
Methylation can increase or decrease the half-life of the drug as seen from the examples of simvastatin where it is increased 2-fold while in case of Etoricoxib addition of methyl group decreases the half-life by more than 10- fold (Figure 2)
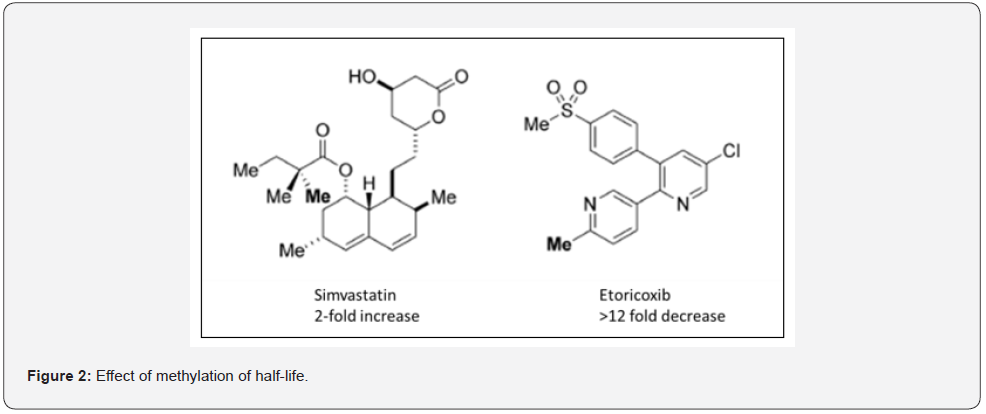
Increase in selectivity
Addition of methyl group may enhance the selectivity of the molecules as can be seen from the example below where selectivity of the molecule towards a particular target increased by almost (Figure 3) 87-fold.

Increase in binding affinity and potency
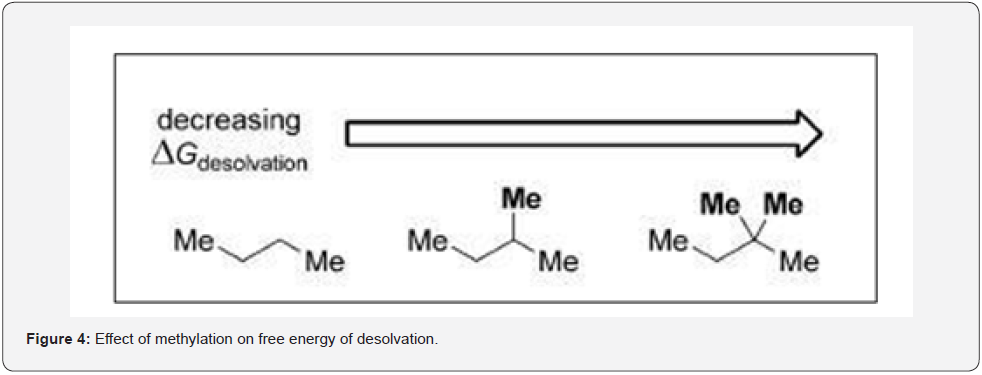
Increased methylation reduces free energy of desolvation required to remove a ligand of solvated water molecules when it transfers from an aqueous environment to the lipophilic environment (Figure 4). This results in increase binding leading to increased potency of the molecule. Jorgensen and co-workers suggested that a single methyl group might boost potency approximately 10-fold if the new methyl group sits nicely in a hydrophobic pocket of the active site. Methylation can bring in optimum lipophilicity in order to cross the phospholipid bilayer of the target organ from an aqueous environment like blood.
This stripping into the organ requires desolvation free energy so by methylating the drug the desolvation energy expenditure is minimized to a greater extent. This process allows an optimum balance between hydrophilic and lipophilic of a drug. This is termed as ligand lipophilicity efficiency (LEE).
Alteration of metabolism
Similar to the interaction between a drug and its biological target, the metabolism of a drug molecule requires it to interact with the active site of the enzyme that catalyzes its metabolism. Steric hindrance is a common strategy used to block or slow a specific metabolic pathway. In this approach, additional atoms are added adjacent to the functional group undergoing metabolism in order to block the interaction of the drug molecule with the enzyme carrying out the metabolic transformation. In many cases, these additional atoms need not be very large. In acetylcholine and bethanechol (Figure 5), the additional methyl group seen on bethanechol prevents the enzyme acetyl cholinesterase from cleaving the ester bond.
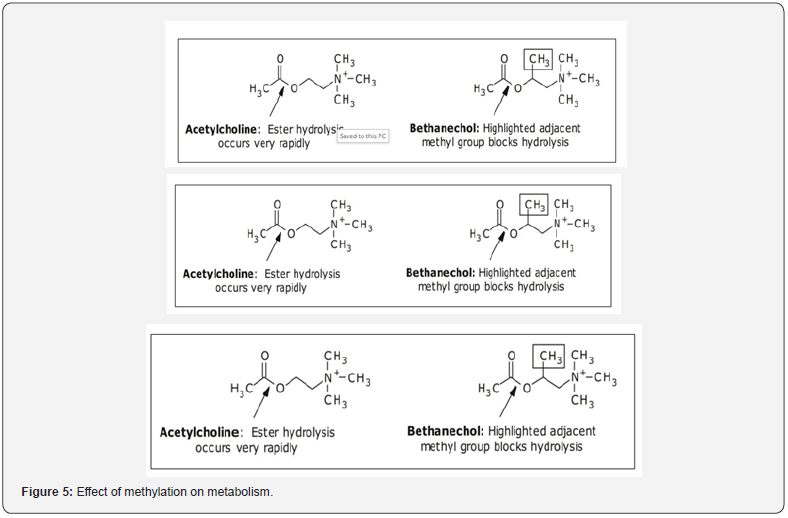
General role in drug design
The methyl group plays an important role in the rational drug design. Introducing methyl into small molecule has become an important strategy of a lead compound optimization. Methyl can modulate the physicochemical, pharmacodynamics and pharmacokinetic properties by ortho effect, inductive effect and conformational effect. It also improves the metabolic stability as a soft metabolic point [2].
Magic Methyl Effect
The ‘magic methyl effect’ refers to the large and unexpected change in drug potency resulting from the addition of a single methyl group to a molecule [3]. Dramatic change in affinity that can be seen with the addition of a single methyl group in just the right place [4]. The methyl group is one of the most prevalent functionalities in biologically active molecules. A study revealed that 10-fold boost in potency with a methyl is seen in 8% of the cases, while a 100-fold difference is seen in 0.4% [3].
Origin of magic methyl effect
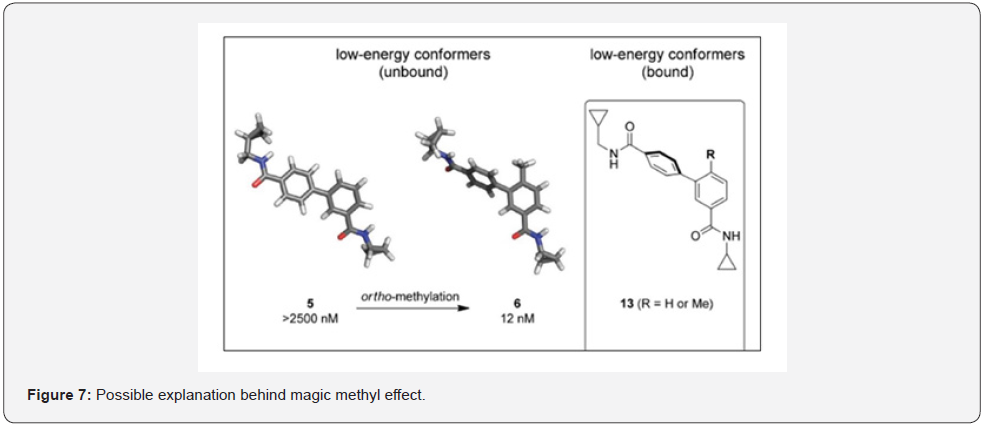
This effect was originally reported when Jorgensen and his co-workers studied set of MAP kinase inhibitors computationally in which just by adding a single methyl group the IC50 value had changed from 2500 nM to 12 nM (Figure 6). The research group observed a torsional twist induced by ortho-methyl group and concluded that this leads to a low energy conformation that closely resembles the conformer observed in X- ray crystal structure of the protein inhibitor complex. They hypothesized that an introduction of methyl group at ortho position induces aryl ring to rotate perpendicular leading to effective binding affinity with the target active site and in turn shows profound increase in potency as shown in Figure 6. It was further noticed that the dihedral angle of the biaryl bond in structure A was 50°C whereas addition of methyl at ortho twisted this angle out to 65°C making it similar to its protein bound conformer. This was hailed to be the reason behind surprisingly beneficial effect on binding affinity (Figure 7).

Some Guidelines for The Strategic Introduction of The Methyl Group [5-14]
After studying this effect scientists have discovered some common strategies which could be used as starting point to introduce methyl group in the design of molecules. These strategies includes:
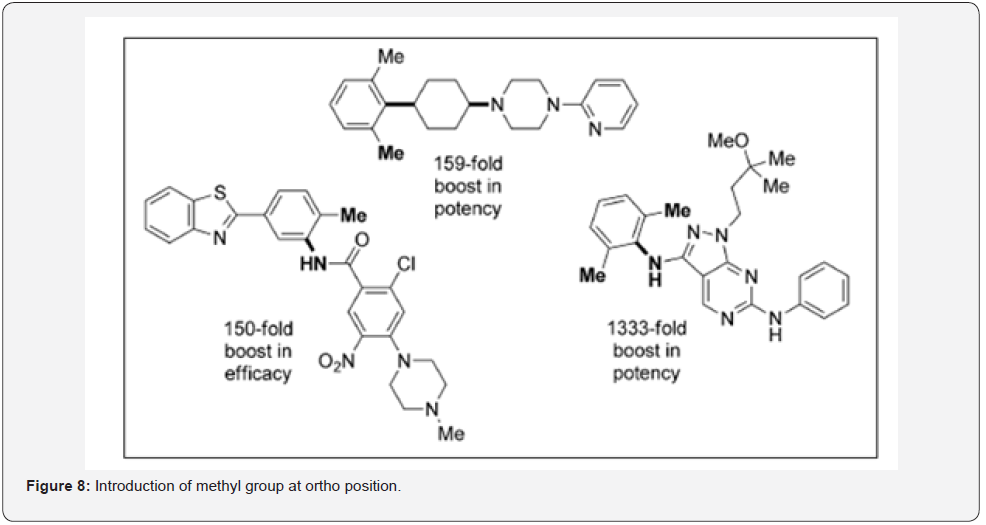
a) Methyl group ortho to a large rotatable substituent on an aryl ring as shown in Figure 8
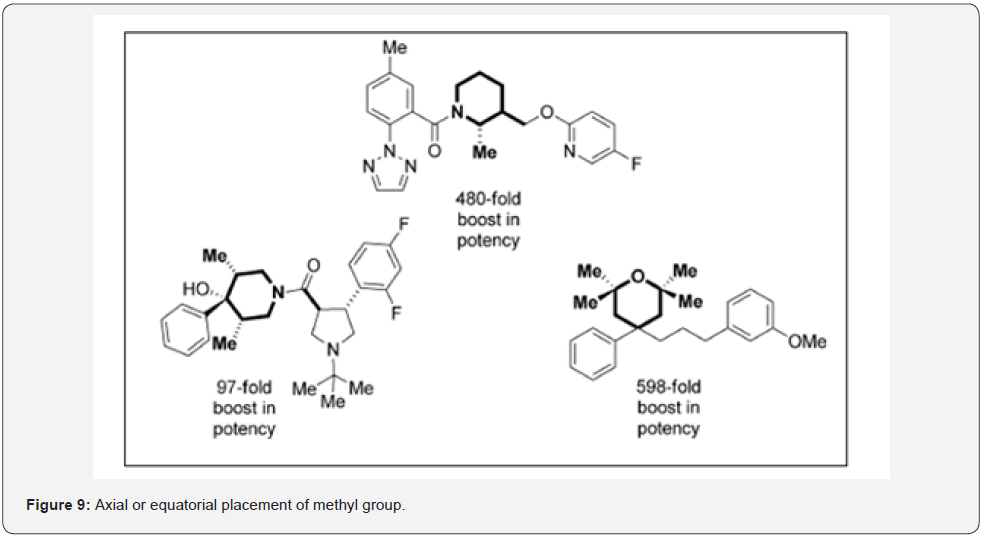
b) On substituted rings where an axial or equatorial preference of substituents can be influenced in Figure 9
c) Between two freely rotatable bonds that are substituted with bulky groups influenced in Figure 10

Conclusion
The biggest gains in the potency in drug design phase will be realized when the interplay of conformational, hydrophobic, desolvation and other effects are cooperatively aligned. The likelihood of discovering an increase in 100-fold boost in potency by installing a single methyl group is extremely low. However, with the advent of synthetic chemistry and availability of easy and simple reactions, methylated analogues of a drug lead merit such an exploration. If simple methylation could result in most effective potent drug with least side effects, then it would be indeed magical.
Click here: https://juniperpublishers.com/gjpps/index.php
Click here: https://juniperpublishers.com/index.php




No comments:
Post a Comment