Anesthesia & Intensive Care Medicine - Juniper Publishers
Abstract
Background: COVID-19 presents a wide spectrum of clinical manifestations from asymptomatic infection to severe pneumonia accompanied by ARDS and multisystemic failure. N-terminal-pro-B-type natriuretic peptide (NT-proBNP) has been proven to be a good predictor of outcomes in patients with ARDS and might be an indicator of severity for SARS-CoV-2 infection, too.
Methods: We conducted a single-centre, retrospective cohort study of critically ill adult patients at the intensive care unit (ICU) with confirmed COVID-19 infection at the clinical centre of Hanau, Germany. Patients were admitted from March 19th to May 25th and were followed-up until June 25th, 2020.
Results: Of 34 patients admitted for COVID-19 to the ICU, 18 (52.9%) survived and 16 (47.1%) died. The majority was male 27 (79.4%). Many patients had cardiovascular diseases 23 (67.6%), chronic kidney diseases 12 (35.3%) and thrombo-embolic events 11 (32.4%). Non-survivors were older than survivors (76.0±7.3 vs. 58.5±14.9 years; p=0.003). In non-survivors we found significant higher levels of d-dimers, creatinine, urea and lactate at ICU-admission compared to survivors. In our population, NT-pro-BNP-Level at ICU-admission could partially help in the prognostication of patients’ hospital survival
Conclusion NT-proBNP may help to categorize the severity of the multisystem COVID-19 disease at admission to the ICU.
Keywords: COVID-19, pro-BNP, ARDS, multi-organ failure, cardiac insufficiency
Abbreviations: COVID-19: Coronavirus disease 2019; pro-BNP: Pro–B-Type Natriuretic Peptide, Acute Respiratory Distress Syndrome: ARDS
Introduction
Coronavirus disease 2019 (COVID-19) has spread rapidly throughout China (Wuhan, Hubei) and almost all countries around the world. Its etiological agent is the coronavirus SARS-CoV-2 [1]. On March 11th, 2020 the World Health Organization (WHO) declared a pandemic. The number of fatalities owing to COVID-19 is escalating and estimated more than 3.1 million patients died world-wide [2].
Patients with COVID-19 present with clinical symptoms of variable severity which might range from detection of virus RNA without symptoms to multiorgan failure and acute respiratory distress syndrome (ARDS) in up to 15% of patients [3,4]. It is difficult to estimate the clinical course in advance and some patients may detoriate rapidly [3].
The immune response to SARS-CoV-2 is known to involve all the components of the immune system that together appear responsible for viral elimination and recovery from the infection [5]. In the late stage of the disease, severe cases suffer from ARDS, metabolic disorders, multiple organ dysfunction (MODS) and coagulation disorders.
Up to now the exact pathophysiological mechanisms responsible for the different clinical courses of COVID-19 patients are not clear. In some patients a severe inflammatory response might lead to a decrease and functional impairment of CD4+ T cells and CD8+ T cells, extraordinarily increased neutrophils, disseminated intravascular coagulation and finally even death [6].
In patients with ARDS of other origin Lai et al. concluded 2017 that N-terminal proB-type natriuretic peptide (NT-proBNP) is a good predictor of outcomes. B-type natriuretic peptide (BNP) was first described in the porcine brain, but BNP in humans originate primarily from the heart’s ventricular myocardium. The secretion of BNP is mediated by the ventricles of the heart in response to excessive stretching of heart muscle cells. Several studies have reported that BNP or NT-proBNP was elevated in patients with ARDS. But only Determann et al. and Park et al. focused on NTproBNP. Accordingly, this peptide may theoretically be used as an indicator of clinical severity for SARS-CoV-2 infection. In a metaanalysis including 13 observational studies and a total of 2248 patients, Sorrentino et al. [4] demonstrated that an elevated NTproBNP level on admission is associated with a worse prognosis in COVID-19 patients [4].
Therefore, we investigated during the first wave of the pandemic in Germany whether the level of NT-proBNP is a possible predictor of mortality in patients with COVID-19.
Materials and Methods
Trial design
We conducted a single-center, retrospective cohort study of consecutive adult patients hospitalized and admitted to ICU with confirmed COVID-19 infection by positive reverse transcription polymerase chain reaction at the clinical center of Hanau, Germany. The hospital is a designated hospital to treat patients with COVID-19 and teaching hospital of the University of Frankfurt, Germany. Patients were admitted from March 19th, 2020 to May 25th, 2020, and they were followed-up until June 25th, 2020. One patient was included despite a negative SARS-CoV-2 PCR due to the typical clinical course and radiological findings in the thorax CT typical for a COVID-19 pneumonia. Clinical information was collected on admission and during ICU stay by attending physicians. This project was performed in accordance with the Declaration of Helsinki and after approval of the local Ethics Committee of the Landesärztekammer Hessen, Frankfurt, Germany (2020-1795-evBO; 28.08.2020).
Data collection
The medical records of the patients were reviewed by a trained team of physicians working in the Hospital of Hanau, Germany, during the epidemic period. Patient data including demographics, medical history, laboratory examinations, comorbidities, complications, procedures, and outcomes (death, need for intensive care unit {ICU}, intubation, mechanical ventilation, renal replacement therapy, ICU- and hospital length of stay {LOS}, and hospital discharge) were collected and analyzed.
Statistical analysis
Continuous variables are presented as mean±SD and median (25%, 75% quartil). Categorical variables are expressed as absolute number of patients and percentage. The mean values for continuous variables were compared using independent group t tests when the data were normally distributed, otherwise, the Mann-Whitney test was used. For pro-BNP we calculated the area under the receiver operating characteristic {ROC} in respect of survival and explored the optimal cutoff value. By means of Kaplan- Meier curves, the survival of patients with pro-BNP below / above the cutoff is illustrated. A p-value less than 0.05 was considered statistically significant. Because of the explorative nature of the study, we did not perform an α-correction for multiple testing, therefore the p-values must be interpreted carefully. All statistical analyses were performed with IBM® SPSS®, version 27 for Windows.
Results
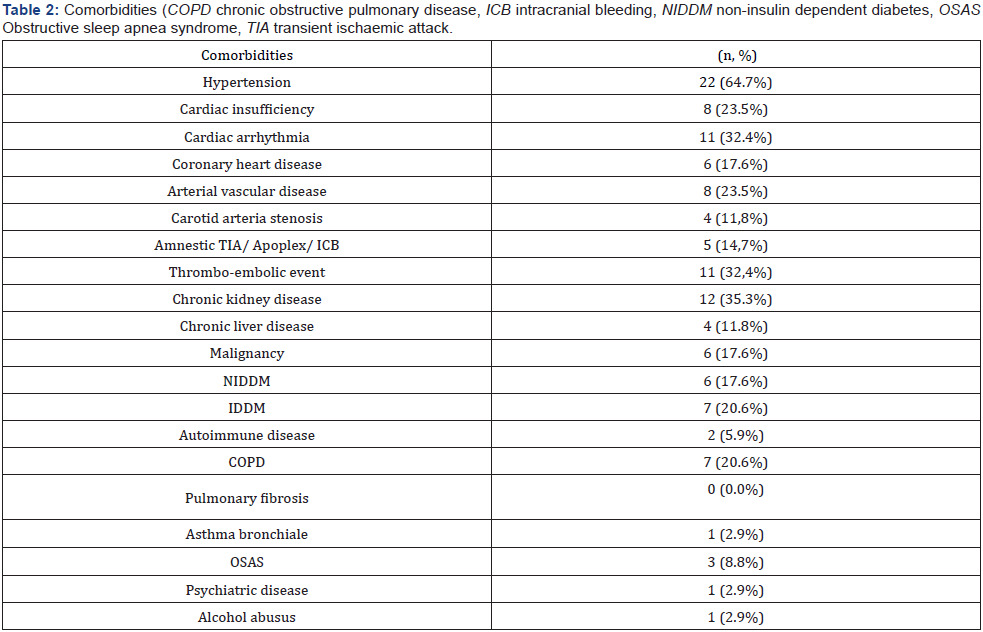
A total of 34 patients were admitted to ICU for COVID-19 during this study period. We had 18 (52.9%) survivors and 16 (47.1%) non-survivors. 27 of them were male patients (79,4%). The age of the total cohort was 67.8±13.9 years. Basic clinical characteristics and respiratory parameters before intubation and extreme values during the first 24 hours of ventilation on the ICU are shown in Table 1. The comorbidities of our patients are demonstrated in Table 2. Most of the patients had cardiovascular diseases 23 (67.6%), chronic kidney diseases 12 (35.3%) and thromboembolic events 11 (32,4%). In the absence of contraindications our patients were anticoagulated slightly elevated to reach a level between prophylactic and therapeutic anticoagulation. Laboratory data are shown at hospital admittance and the first value measured on intensive care unit (ICU) (Table 3).
All 34 patients were admitted to the ICU due to progressive respiratory failure. We treated 28 (82.4%) patients with highflow oxygen therapy, 24 (70.6%) patients were intubated and invasively ventilated, 22 (64.7%) were proned and only 3 patients (8.8%) were non-invasively ventilated. Many patients (28, 82.4%) showed laboratory or clinical signs of kidney injury, 15 (44.1%) patients developed a new AKI, 9 (26.5%) an acute on chronic kidney injury and only 4 (11.8%) patients chronic kidney injury. 19 patients (55.9%) needed renal replacement therapy at any time during their ICU stay whereof only 4 (11.8%) had pre-existing end-stage renal disease.
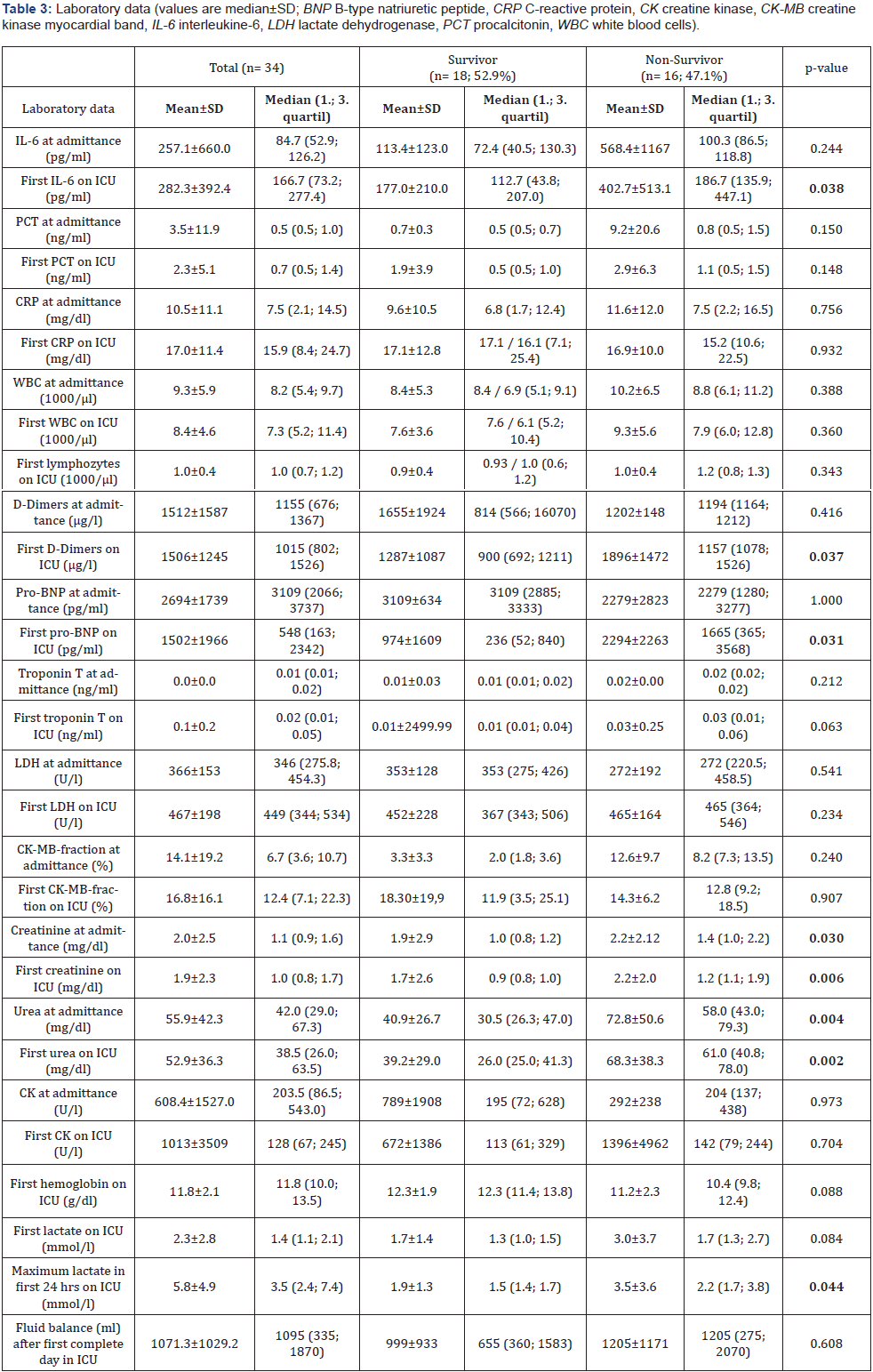
From our 34 ICU patients, almost half (16, 47.1%) died during their hospital stay. Non-survivors were older than the survivors (75.5±7.3 vs. 60.9±14.9; p=0.003); the youngest non-survivor was 64 years old; the oldest survivor was 88 years old. The laboratory data demonstrated for the non-survivor group at admission on ICU increased levels of IL-6, abnormal levels for d-dimers, pro-BNP, creatinine, urea and lactate compared to survivors. The survivors had a higher paO2 level and Horovitz index before intubation. The total duration of high-flow nasal cannula oxygenation therapy (NFHC) was longer for the survivors.

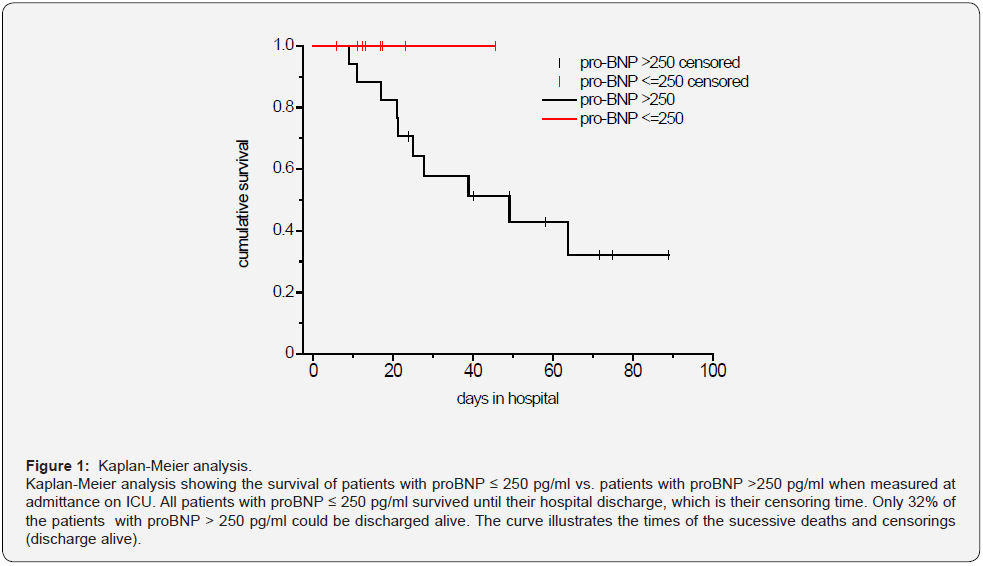
Because of the described elevation of pro-BNP in ARDS and its potential use as a prognostic marker in ARDS we looked in more detail at the pro-BNP level at admittance on ICU and survival. We calculated the receiver operating characteristic (ROC) curve for pro-BNP in respect of survival, which resulted in an AUC of 0.760 (p = 0.007). This is a hint, that pro-BNP is a significant predictor of the survival. We received a pro-BNP value of >244 pg/ml as the optimal cut-off value for our sample. With this cutoff value the sensitivity is 1 and the specifity 0.533. As the adjacent cutoff-value is 252, we determined 250 as optimal cutoff.
The Kaplan-Meier analysis (Figure 1) shows the survival of patients with proBNP ≤ 250 pg/ml vs. patients with proBNP > 250 pg/ml. All patients with proBNP ≤ 250 pg/ml survived until their hospital discharge, which is their censoring time. Only 32% of the patients with proBNP > 250 pg/ml could be discharged alive. The curve illustrates the times of the successive deaths and censorings (discharge alive).
Discussion
In this retrospective analysis of 34 ICU patients with respiratory insufficiency due to a COVID-19 infection during the first part of the pandemic in a municipal hospital in Germany we found clinical courses similar to those in other studies [7,8]. Severe respiratory failure in patients with SARS-CoV-2 infection is only one sign of the multisystem inflammatory syndrome [9-11], which is consistent with the high rate of patients with renal failure and the need for renal replacement therapy in more than half of the patients [12,13]. The high rate of multiorgan failure together with the increased age of our patients may at least partially explain the high mortality of our patients which is comparable to data from other studies with COVID-19 ICU patients [14].
More than two third of our patients were invasively ventilated and only a minority was non-invasively ventilated because at that time - during the “first wave” in Germany - we worried about an increased risk of transmission for the ICU staff with the use of noninvasive ventilation. Accordingly, we suggested a reduced risk of transmission of SARS-CoV-2 when patients were intubated timely. In the meantime, it has been demonstrated that is reasonable, safe and recommended in the guideline to try nasal-high flow oxygen and non-invasive ventilation in patients with respiratory insufficiency due to COVID-19 when patients are closely monitored [2,15-18].
Very soon after the first patients with COVID-19 had been treated it was recognized that there is an increased risk for thrombo-embolic events in these patients due to an inflammatory alteration of the endothelium and an inflammatory pro-coagulatory state [19,20]. It was difficult and potentially misleading to count the number of thrombo-embolic events in our patients because we did not screen systematically for these events as other groups did [20].
The major finding in this - small group - of patients with COVID-19 is that an elevated pro-BNP level on admission to the ICU shows the tendency of a worse prognosis.
Many clinical data described a cardiovascular manifestation induced by this viral infection especially in critical ill patients. Acute myocardial injury manifested mainly by elevated levels of high-sensitive troponin I, and arrhythmias have been detected [9,21]. Guo et al. [3] reported in their study among 187 patients with COVID-19, 52 (27.8%) exhibited myocardial injury as demonstrated by elevation of troponin T (TnT) levels, and the mortality was markedly higher in patients with elevated TnT levels than in patients with normal TnT levels (59.6% vs 8.9%). The authors suggest that myocardial injury due to the inflammation might play a major role in the clinical detoriation of COVID-19 patients and that those patients with elevated troponin T levels (27.8% of patients) had more malignant arrhythmias and a higher mortality [3]. Consistent with our results of increased levels of pro-BNP in non-survivors they found a correlation between elevated pro-BNP and troponin T levels [3].
In our group of non-survivors TnT levels were a bit higher at admission to the ICU (0.03 ± 0.25) and proBNP was significantly higher in the non-survivor group (2294±2263; p =0.031) compared to the survivors. The comorbidities of our patients represented mostly cardiovascular diseases, hypertension on top of all (64.7%). Alvarez-Garcia et al. [5] pointed out that patients with a history of heart failure (HF) hospitalized for COVID-19 face nearly 3 times the risk of mechanical ventilation and twice the risk of mortality compared with patients without HF [5]. Similar to our results Sorrentino et al. demonstrated a correlation between increased pro-BNP levels and severity of COVID-19 disease but in contrast in their study the pro-BNP levels in non-survivors were already increased at admittance to hospital compared to survivors [4]. In our investigation patients with COVID-19 had also elevated levels of proBNP at admission to the hospital. Thus, we learned from several investigations during the pandemic that predictors of a fatal outcome in COVID- 19 cases included age, the presence of underlying diseases, the presence of secondary infection and elevated inflammatory indicators in the blood [5,14,22,23]. Although the high accuracy of NT-proBNP is already established in the diagnosis of acute heart failure, the prognostic value of this marker for patients with COVID-19 remains uncertain [4].
Our study has several limitations. Only 34 patients with COVID-19 were included during the first wave in Germany, and a larger randomized cohort study is needed to verify our conclusions. Unfortunately, we could not provide more information regarding cardiovascular complications as e.g., cardiovascular ultrasound or detailed hemodynamic monitoring. Due to the restricted options and the increased efforts in the isolation ward data was not complete in all patients.
Last but not least, the data of this retrospective study permit a preliminary assessment of the clinical course and outcomes of patients with COVID-19.
The causes of death may involve multiple organ dysfunction in most cases, and it is difficult to differentiate the myocardial injury as the main and direct cause in an individual case. Long-term observation and prospective study design on the effectiveness of treatments are needed. We still have to wait for long-term results after surviving Covid-19 disease [24].
Conclusion
Many critically ill patients with COVID-19 pneumonia suffer from multi-organ dysfunction including cardiac insufficiency. We concluded the measurement of specific laboratory data as NT-proBNP may help to categorize the severity of the COVID-19 disease at admission to the ICU.
To Know more about Anesthesia & Intensive Care Medicine
Click here: https://juniperpublishers.com/jaicm/index.php
Click here: https://juniperpublishers.com/index.php




No comments:
Post a Comment