Biotechnology & Microbiology - Juniper Publishers
Abstract
As coronavirus disease 2019 (COVID-19) infections and related deaths continue to negatively impact the world, methods for adequate personal protection and hygiene remain a crucial topic of discussion. In the United States of America, current recommendations related to hand hygiene include washing hands with soap and water or to use a hand sanitizer with approved FDA ingredients when soap and water are not readily accessible. Today, a novel technology that utilizes a proprietary formula consisting of reengineered amorphous colloidal hydrated silica in combination with minimal amounts of agency approved inert and active ingredients has proven to not only be effective on contact but continues to protect long after it has dried. This technology was developed over a decade ago and over that period has proven to be both safe and effective. As the world is faced with yet another global pandemic the need for proven biocidal solutions are vital. The purpose of this short literature is to lay out the facts associated with both alcohol-based and non-alcohol-based hand sanitizers and present the findings demonstrated by microSURE™ Hand and Skin Sanitizer in a bio safety level-3 (BSL-3) laboratory, when tested directly against Severe Acute Respiratory Syndrome coronavirus 2 (SARS Co-V2), the virus responsible for causing (COVID-19). The details presented throughout this paper will provide insight as to why Benzalkonium chloride (BZK) must not be overlooked when deciding on the best method for protecting your body from harmful microbes and protecting your skin from potential damage.
Keywords: Coronavirus; Benzalkonium chloride; Hydrated silica; Colloidal silica; Amorphous silica; Antimicrobial; Hand sanitizer; Hand hygiene
Abbreviation: BZK: Benzalkonium Chloride; CDC: Center for Disease Control and Prevention; COVID-19: Coronavirus disease 2019, FDA: United States Food and Drug Administration; SARS-Co-V2: Severe Acute Respiratory Syndrome Coronavirus 2
Background
Over the past two decades, society has had to combat three extremely pathogenic coronaviruses that have all carried detrimental effects on the human population. [1,2] Coronaviruses are a family of viruses that are understood to cause illnesses which can vary from the common cold to severe diseases such as Middle Eastern Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS). [1-4] Current literature suggests that each of the mentioned coronavirus outbreaks originated from an animal source, the first (SARS-CoV) is said to have transmitted from civet cats in 2002, (MERS-CoV) is said to have transmitted from camels in 2012 and the novel (SARS-CoV2), the virus responsible for coronavirus 2019 (COVID-19) is said to have been transmitted from bats in 2019[4-6]. Both of the SARS associated coronaviruses have been linked to originating in China and MERS in Saudi Arabia, however all three members of the coronavirus family began to rapidly infect individuals throughout the globe and have collectively been responsible for hundreds of thousands of deaths [7]. Currently, there are no approved vaccines or antiviral drugs for the (SARS Co-V2) virus and because of this, having the ability to control and or contain the virus in order to limit dissemination is vital. It is for this very reason why proven solutions for personal protective equipment, sanitization, cleaning, and disinfecting are eminent means of safety measures.
Although members of the coronavirus family share several commonalities, this literature will focus primarily on (SARSCoV2). Transmission of the virus is predominantly spread from human to human via respiratory droplets, which are naturally expelled as an infected individual sneezes or coughs [1-3]. Recent studies have proven that the virus has the ability to persist on inanimate surfaces such as plastic, stainless steel and cardboard and can remain on these surfaces for days at a time [3]. Other studies have concluded that the novel virus has the ability to linger on human skin far longer than the commonly known flu virus can, stating that the virus has the potential to remain on human skin for up to 28 days [8]. Benzalkonium chloride (BZK) is an active ingredient present in many of today’s consumer products. The use of BZK was first reported as a promising skin disinfectant in 1935 [9]. Since then its use has evolved to being much more than just a skin disinfectant. BZK is currently being utilized not only in personal care products such as hand sanitizers, soaps, lotions, and shampoos, but it is also found in spray disinfectants, pharmaceutical products such as eye drops, skin and wound antiseptics, mouthwashes, surgical disinfection products, burn treatments and more. One of the major actions of BZK relates to its antimicrobial capabilities, the biocidal actions of BZK create a dissociation of the unwanted microbe’s membrane lipid bilayers and stimulates the leakage of cellular contents, therefore compromising its permeability and eliminating its presence [10].
Research Based Evidence
BZK has been proven effective against bacteria, viruses, fungi and protozoa [11]. The reason for its widespread use of application aside from its antimicrobial efficacy is based on the fact that it is easier on the skin than the majority of other skin disinfectants, especially when being compared to alcohol. Unlike alcohol, BZK can be used directly on open skin or an open wound without causing damage to the wound bed or creating a painful sting and or burning sensation. When it comes to hand sanitizers, there has been a lot of debate as to whether an alcohol-based sanitizer or non-alcohol-based sanitizer is the better option.
In 1998, a study using non-alcoholic based sanitizer with benzalkonium chloride as the active ingredient was completed via FDA performance standards and determined that the benzalkonium chloride-based sanitizer performed better than alcohol-based hand sanitizer after repeated use [12]. It wasn’t until recently that BZK has begun to once again become a common topic of discussion as it relates to the current ‘coronavirus disease 19’ (COVID-19) pandemic caused by ‘Severe acute respiratory syndrome coronavirus 2 (SARS Co-V2) and the measures society is taking in order to eliminate the risk of spreading or contracting microbes responsible for causing illness and disease. Of late, there has been a need for more BZK studies and research regarding hand sanitizers, as the amount of data available is still limited when compared to the more commonly used alcohol-based products.
As history is often known to repeat itself, newer studies continue to prove what previous research pointed out, and that is that benzalkonium chloride hand sanitizers demonstrate greater effectiveness than alcohol-based hand sanitizers. For example, in an infection control study performed by Bondurant et. al. to evaluate the effectiveness of BZK as the active ingredient in reducing transient skin contamination with staphylococcus aureus in health care workers, as compared to the effectiveness of an ethanol-based hand sanitizer, research found that the benzalkonium hand sanitizer significantly reduced staphylococcus aureus versus an ethanol sanitizer and also concluded that BZK had greater skin presence and persistence [13]. This same research study was able to reference multiple experiments which have confirmed that BZK- manufactured products demonstrated ‘persistent antibacterial efficacy’ even up to four hours after bacterial contact with skin, as opposed to alcohols efficacy duration, which has only been documented to reach around 10 minutes maximum [13]. Although efficacy is extremely vital when it comes to antimicrobial properties associated with hand sanitizer, the other crucial aspect is the affect it has on people’s skin and overall health. It is important to note the differences in the amount of active ingredient present between each set of hand sanitizers, BZK is usually only around .13% of the final end product, while alcohol normally exists in excess or 60% or greater of the final end product. Today, society seems to be much more focused about the ingredients present in consumer products, as well as living a health-conscious and safer lifestyle. There has been an increase in the number of vegetarians, vegans and plantbased diets, there has been an increase in the number of health club memberships, and social media has been helped educate people on the different changes they can make to live a more health-conscious lifestyle.
With that said, appearance and personal care has also become very important and because individuals have become so concerned with what products may harm their bodies, the use of alcohol-based hand sanitizers is no longer the only go to option. Many big-name hand sanitizers use alcohol as an active ingredient and the reasons for this are quite clear. Alcohol is ridiculously cheap, it is effective at eliminating many of the common germs responsible for causing the spread of infection directly on contact, and it is widely available. In addition to these factors, alcohol has been around for a much longer time period and because of this it would be safe to assume that it has become widely accepted by society as the ‘only’ reliable option. Unfortunately, alcohol is not as safe as many people believe it is and there have been several studies which prove this.
Research has proven that the continued use of alcoholbased hand sanitizers is responsible for several underlying skin reactions. These reactions include dryness, itching, irritation, cracking and even bleeding. [14] To most individuals, the notion of dry or cracked skin may not seem to be that serious of an issue, but this perception drastically changes when realizing the detrimental health effects that can arise from these ‘little’ issues.
The cracking and dryness of the skin leads to changes in the skin flora, resulting in more frequent bacterial colonization and bacterial susceptibility, particularly by staphylococcus aureus and gram-negative bacilli. [15] This means that as consumers continue to use alcohol-based hand sanitizers, over time, as the skin dries and the more alcohol-based sanitizer you use increases, so does the risk for infection. Therefore, the product that was originally being used to help eliminate the risk of infection, instead becomes the culprit responsible for inviting infection.
Current Guidelines
Current Center for Disease Control and Prevention (CDC) guidelines suggest that alcohol-based hand sanitizer with a high concentration and or antiseptic hand soap for hand washing are the best options for hand hygiene. [16] Alcohol has been proven to be effective at killing bacteria when concentrations are between 60% and 90% and works mainly because of its ability to denature proteins. [5,9] However, studies have actually shown these higher concentrations of alcohol essentially lose their effectiveness because water is needed as an adjunct in order for maximum potency to be achieved [17]. In 2016, the United States Food and Drug Administration (FDA) removed 19 antimicrobial ingredients from the list of allowed consumer products, BZK was not one of them [18]. Although BZK was not removed from the FDA’s allowed antimicrobial list, The CDC notes that the reasons for its statements regarding high concentration alcohol-based hand sanitizers instead of BZK or other various quaternary ammonium products is because such products necessitate further studies [17,18].
Materials and Methods
In September of 2020 test results of microSURE™ Hand & Skin Sanitizer directly against the SARS Co-V2 virus were completed and released. This testing was accomplished to demonstrate residual efficacy of the solution and was conducted in a bio-safetylevel 3 (BSL3) laboratory. A very concise protocol was followed, as indicated below.
Petri dishes were coated with the following treatments:
a. diH20- deionized water (Negative treatment control)
b. MicroSURE™, formulation of IP material and Benzalkonium chloride (BZK)
The coating procedure was as follows:
a. 100mm and 60mm petri dishes were used to create a sandwich to expose virus to treated surfaces
b. The inner bottom surface of 100mm dishes were filled with 1.0 ml material, to cover the surface. The bottom of a 60mm dish was placed in the material, exposing the bottom to the material.
c. The petri dishes sat for 10 minutes and then the coating materials were removed, and the dishes allowed to dry.
d. Petri dishes were packaged in pairs with the 60mm dish inside the 100mm dish with the lid on the 100mm dish and stored at room temperature.
e. Coated petri dishes were then subjected to viral inactivation testing at several different times points after coating: 3 hours, 1 day, 2 days and 8 days.
Materials were transferred to the BSL3, and inactivation tested as follows at 4 different time periods after coating–3 hours, 1 day, 2 days and 8 days:
a. Quintuplate dish sets were transferred into the BSC.
b. 20 μl SARS CoV2 virus (5e6 FFU/ml, so total of 1e5 FFU) placed on treated 100mm petri dish.
c. The 60mm petri dish was placed on top, sandwiching the inoculum between the dishes.
d. The dishes were incubated at RT in humidified box for 30 minutes.
e. The dishes were separated and rinsed 5x with 0.5 ml of infection medium (DMEM with 2%FBS and antibiotics).
f. The 0.5 ml of infection media was transferred to sterile tubes and tested in a FFU assay.
FFU assay (immunostain for focus-forming units)
a. To each well of a 96-well plate seeded with Vero cells, add 50 μl neat media (from coverslip rinsing) and dilute 10-fold to 10-5
b. Incubate plates for 1 hr and then overlay with 50 μl Methycellulose medium
c. Incubate approximately 24 hr at 37°C, 5% CO2
d. Remove media, washing with PBS and fix plates with 80:20 MeOH: Acetone
e. Remove plates from BSL3 following approved protocol
f. Immunostain plates with anti-SARS-CoV-2 Spike mAb, 1C02, using anti-human IgG-HRP to visualize FFU. Count FFU comparing diH2O-treated to test material-treated coverslips.
Results
Treatment of the surfaces by the test material microSURE™ significantly reduced the amount of infectious material, SARSCoV- 2, at all-time points after coating compared to the control, diH2O-treated surfaces, with a 30minute virus contact time. These results, which show a >3-4 log reduction at all time periods, is both statistically significant (p<0.05 t-test, post hoc Mann- Whitney test), as well as biologically relevant (Figure 1).
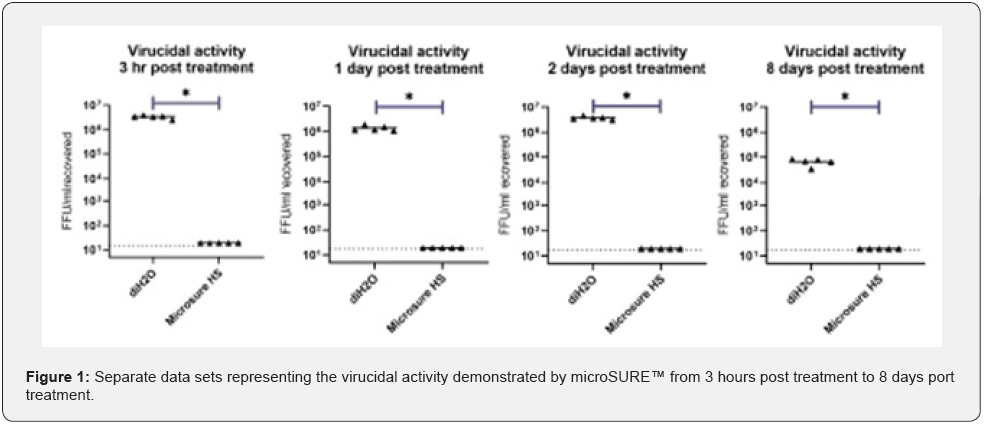
Conclusion
The microSURE™ Hand and Skin Sanitizer proved to be efficacious against the novel SARS CoV-2 virus throughout the entire 8-day testing period. Based on this testing, this solution that consists of a proprietary formulation in combination with BZK is a superior alternative to alcohol-based solutions as it relates to efficacy, specifically virucidal residual efficacy. Overall, when deciding on the best hand sanitizer to use, it is important that the product is both efficacious and as safe as possible for the skin. Although both BZK and alcohol have positive effects when being used as an active ingredient in hand sanitizer, there are several factors which differentiate the two. Alcohol has been around for a much longer time period and has been studied in much greater detail because of this. Alcohol is also readily available, inexpensive, and widely accepted by the general public because there was never enough literature to suggest a superior alternative, until now. As mentioned previously, BZK has been around for over 90 years, yet the amount of research and experiments were never at a level like that of alcohol.
Yet, even with limited data, the FDA still did not remove the ingredient from its approved list of antimicrobial active ingredients for accepted use in hand sanitizers, and the reasons for this are obvious. BZK has been proven to be more effective than alcohol as an active ingredient throughout numerous studies. In comparison to alcohol, BZK has specifically been proven to combat some of the more problematic bacterial infections, such as those associated with staphylococci and gram-negative bacteria. Furthermore, when looking the results seen with the microSURE™ Hand and Skin Sanitizer with BZK, this solution not only demonstrated superior efficacy against the novel coronavirus, but it also demonstrated a residual efficacy that is unmatched by any other alcohol-based sanitizer and other sanitizers with BZK as an active ingredient. In terms of health effects, the skin reactions documented with alcohol-based sanitizers tend to be much more common and serious when compared to BZK. Although current CDC guidelines suggest using a high concentration alcohol-based hand sanitizer in an attempt to prevent bacterial infection, countless studies have been conducted and prove that high concentration alcohols are not potent by themselves and that alcohol-based hand sanitizers come with the increased risk for skin reactions to occur. The continued use of alcohol-based hand sanitizer has been well documented, and the negative affects it has on skin can be debilitating when it comes to providing a reliable defense against infection. This is because as consumers continue to use this type of hand sanitizer, the skin dries and cracks, altering the normal skin flora and therefore becomes susceptible to unwanted and harmful microorganisms. The important concept to remember is that there are other available options besides alcohol-based hand sanitizers. Time and time again, BZK-based hand sanitizers have been proven to be both efficacious and safer on skin than alcohol-based sanitizers. New literature is beginning to focus more on BZK and the hope is that as studies continue to become available and findings are more publicized, the general public will learn that there is another alternative to using alcohol-based hand sanitizers and this alternative is one that has continues to show preeminence over the commonly used alcohol based hand sanitizers currently available.
To Know more about Biotechnology & Microbiology
Click here: https://juniperpublishers.com/aibm/index.php
Click here: https://juniperpublishers.com/index.php




No comments:
Post a Comment