Open Access Journal of Toxicology - Juniper Publishers
Abstract
Introduction: Acquired or toxic methemoglobinemia is the result of exposure by swallowing, inhalation or dermal absorbtion of medicines or non-drug chemicals with oxidizing properties. The main objectives of the study were to highlight the main causes of methemoglobinemia in children, to identify the associated factors and their influence on the clinical form of the disease as well as the response to antidote treatment with methylene blue.
Methods: All patients with acute toxic methemoglobinemia hospitalized over a period of six years were included in the study, the inclusion criteria being: age < 18 years, suggestive symptomatology (generalized cyanosis, asthenia, dyspnea) and methemoglobin values >3%.
Results: 82 patients with acute toxic methemoglobinemia were identified of which the majority (94%) were between 0-5 years. 69 cases were secondary to exposure to nitrates from water used to prepare food. The severity of methemoglobinemia was significantly higher among anemic and dehydrated patients. All patients received antidote treatment with methylene blue one or more administrations, and all had a favorable outcome.
Conclusions: Acute toxic methemoglobinemia is mainly observed in children up to 5 years of age, the majority being infants. The main cause of it in children is exposure to exogenous substances among which nitrates that contaminate well water occupy the first place. Age under 1 year, hemoglobin values lower than 11g/dl, and acute dehydration syndrome represent risk factors for severe forms. Methylene blue is the treatment of choice for acute toxic methemoglobinemia in children and its administration is effective in the majority of cases.
Keywords: Acute toxic, Methemoglobinemia, Children, Methylene Blue
Introduction
Acquired or toxic methemoglobinemia is defined as an increase in methemoglobin blood levels above 1%, as a result of exposure to medicines or chemicals with oxidizing properties. The medicinal etiologies of toxic methemoglobinemia include, acetanilide, phenacetin; local anesthetics (benzocaine, lidocaine, prilocaine, and articaine), chloroquine, dapsone, flutamide, metoclopramide, nitric oxide, nitroglycerin, nitroprusside of sodium, antibiotics of the class of nitrofurans and sulfonamides, sodium nitrate, silver nitrate, silver sulfadiazines, antiepileptics (sodium valproate, phenytoin), phenazopyridine, sulfasalazine, and zopiclone [1]. Among non-drug chemicals that can produce methemoglobinemia, the most common include aniline-based paints, silver nitrate, naphthalene, trinitrotoluene, nitrobenzene, Vicia faba, nitrates and nitrites from well water or contaminated vegetables, paraquat, phenol, or fires [1].
Acute toxic methemoglobinemia in children can be triggered by any of the above-mentioned xenobiotics It has the potential to cause severe injury or death but if recognized early, patients can significantly benefit from antidote treatment. The antidote of methemoglobinemia is methylene blue a thiazine coloring agent, which in the presence of NADPH-dependent methemoglobin reductase and nicotinamide adenine dinucleotide phosphate causes the reduction of methemoglobin and its subsequent transformation into hemoglobin. Antidote treatment with methylene blue is commonly indicated in all symptomatic patients or cases where methemoglobin concentration is above 30% [1].
In Romania acute toxic methemoglobinemia in children was recognized as a public health problem for many years [2]. A national report of the Ministry of Health published in 2012 revealed that Romania is characterized by the existence of numerous rural territories with frequent and important nitrogenous substances contamination [2]. Nitrates contaminate the soil from where water infiltrates into artisanal wells existing in rural areas. The toxicity of these salts is due to their transformation into nitrites under the action of bacteria at the level of the soil or gastrointestinal tract of the child. Nitrites exert a direct oxidant effect on hemoglobin, which subsequently loses its physiological capacity for carrying oxygen. The level of nitrates in the water depends on several elements, and hence it is difficult to quantify soil permeability, well depth < 15 m, agricultural activities, and inadequate measures for eliminating organic waste, making it difficult to identify populations at risk based only on geographic origin [3]. The World Health Organization has regulated the maximum permissible concentration of nitrates (NO₃) in drinking water to <50 mg/L [4]. Various local research has been proven that in countries like Romania, a country with poor infrastructure in rural areas, exposure to nitrates remains the main cause of acute toxic methemoglobinemia in children [5].
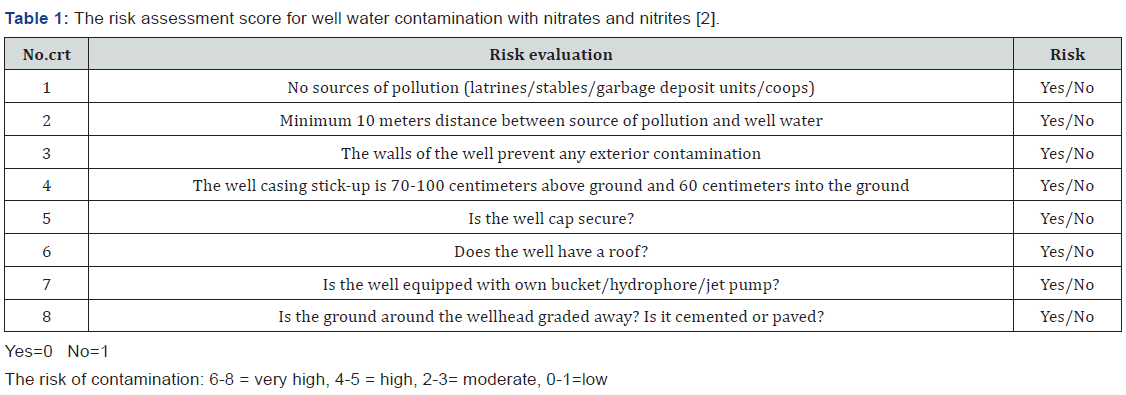
To come to health care specialists’ aid, the Ministry of Health of Romania has developed a score for assessing the risk for well water contamination with nitrates and nitrites (Table 1) [2].
The research presented in the study details a retrospective study including cases of acute methemoglobinemia that were hospitalized and treated at the Pediatric Poisoning Center of the Emergency Clinical Hospital for Children”Grigore Alexandrescu” in Bucharest Romania and analyzed , for a period of 6 years. The main objectives of the study were to highlight the main causes of acute methemoglobinemia in children, to identify the associated factors (age and associated pathology), and their influence on the clinical form of the disease as well as the response to antidote treatment with methylene blue. The study design will be of significant help to global researchers and pharmaceutical firms looking forward to analyze methemoglobinemia in study populations in their respective countries.
Methods
All patients with acute toxic methemoglobinemia hospitalized at the Pediatric Poisoning Center of the Emergency Clinical Hospital for Children “Grigore Alexandrescu” in Bucharest, Romania between January 01, 2015, and December 31, 2020, were included in the study. The inclusion criteria were age < 18 years, patients with suggestive symptomatology (generalized cyanosis, asthenia, dyspnea, and altered neurological status), and methemoglobin values > 3%. For this purpose, we used the computer data base of clinical consultation sheets and medical reports upon discharge of patients using for searching the ICD 10 code of diagnosis for acute toxic methemoglobinemia. The following data were analyzed: medical history data, demographic characteristics, data provided by clinical examinations from patient hospitalization and daily evaluations during hospitalization, the presence of methemoglobinemia (measured by spectrophotometric method), and associated conditions. The therapy regimens provided to the patients and the outcome of each patient were also analyzed, including the duration of hospitalization, the need to repeat the dose of the antidote, and admission to the intensive care department. The data obtained were statistically processed using the IBM® SPSS® Statistics 20program, considering significant results of statistical probability (p) less than 0.05.
Results
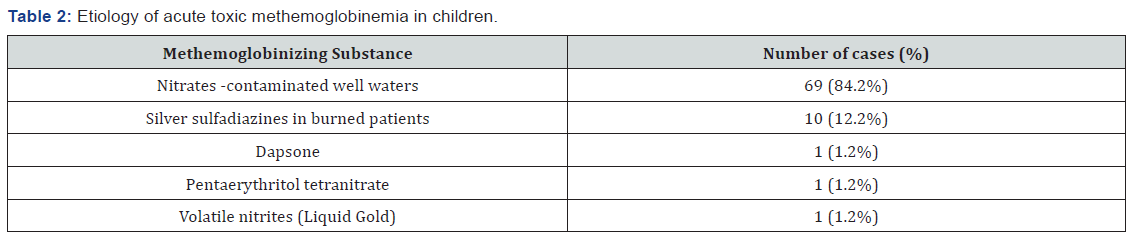
During the mentioned period, 82 patients with acute toxic methemoglobinemia were identified representing a percentage of 1.58% from the total of 5122 patients diagnosed with acute intoxication in the Pediatric Poisoning Center in Bucharest. Etiology Regarding etiology: clinicians ascribed 69 cases as secondary to exposure to nitrates from well water used to prepare food. The risk assessment score for well water contamination with nitrates and nitrites (Table 1) was used, all the 69 children having a risk score between 6 to 8. Ten cases were secondary to topical exposure to silver sulfadiazine used to treat severe burns, two cases were instances of medicines poisoning: one patient had accidental acute poisoning with pentaerythritol tetranitrate, (a coronary vasodilator) and another patient was undergoing chronic treatment with dapsone. In the study group, a case of acute toxic methemoglobinemia was identified, wherein the patient was a chronic consumer of drugs of abuse and was hospitalized following an overdose of volatile nitrites by inhalation (cigarettes soaked with a street substance known as “Liquid Gold”) (Table 2).
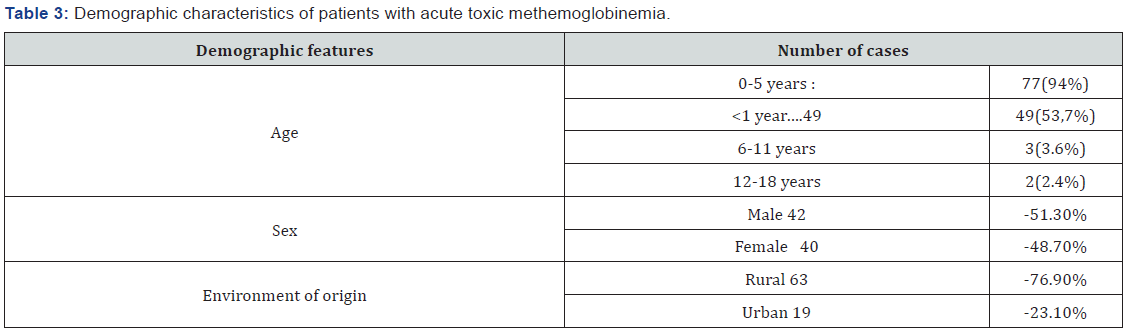
The average age of the patients was 23.11 months, and the median age was 6 months. The majority of patients were under 5 years old: 77(94%), The infants subcategory (children aged under 1 year) within this age group was most represented with 49 patients (53.7% out of the total of cases). At the same time the number of cases was much lower for other age groups (Table 3). Cases among patients of rural origin were significantly higher than those from urban areas (63 versus 19 cases, p<0.05). In addition, rural origin was significantly associated with exposure to nitrates contaminated well water (binomial test, p<0.0001). The study group included 42 boys and 40 girls, the difference in patient sex being insignificant (Table 3). The most common route of exposure was the oral route (87%), followed by cutaneous (12.2%), and inhalation (0.8%). In the majority of cases (85.3%), toxic exposure was accidental.

The mean methemoglobin concentration in the study group was 41.53%, with values ranging from 6.6% to 87% (Table 4). The mean value of methemoglobin for cases of exposure to nitrates -contaminated well water was 41.5%, and it was 39.2% for cases involving topical exposure to silver sulfadiazine, used in patients with severe burns. The methemoglobin values were: 41.9% for case of accidental acute poisoning with pentaerythritol tetranitrate, 44.2% for voluntary inhalation, with new substance of abuse “Liquid Gold “and 6.6% for a patient undergoing dapsone chronic treatment. Since the number of cases caused by exposure to dapsone, pentaerythritol tetranitrate, and new substance of abuse “Liquid Gold “(volatile nitrites) was small (one per etiology), we could not perform a statistical analysis of methemoglobin concentrations in these etiologies. Regarding the mean concentrations of methemoglobin secondary to exposure to nitrates contaminated well water and silver sulfadiazine, the difference was not statistically significant.
Clinical Manifestations:
Upon admission to the toxicology department, all patients showed clinical manifestations with different grades of severity. Clinical manifestations are summarized in Table 4.
The lowest concentration of methemoglobin recorded in this study was 6.6%. Regardless of the methemoglobin concentration, all patients experienced altered general conditions and cyanosis. For values between 6.6% and 20%, other clinical manifestations besides cyanosis, including dyspnea and agitation, were observed in 2 of the 10 identified cases (20%). The majority of patients with methemoglobin values between 20% and 50%, had dyspnea (24.4%), tachycardia (19.5%), psychomotor agitation (31.7%), and drowsiness (12.2%). Those with methemoglobinemia between 51% and 70% showed clinical manifestations, with a higher frequency of dyspnea (37%), tachycardia (29.6%), and altered sensory function (25.9%). Two patients in this group (7.4%) experienced generalized tonic-clonic seizures. These two patients had not preexisting neurologic disease and were 3 months respectively 7 months old. Seizures were ceased after 5 mg diazepam intrarectal administration and required one dose of 1 mg/kg of methylene blue.
All patients with a methemoglobin value above 70% experienced tachyarrhythmia, dyspnea, and impaired sensory function (Table 4). Evolution was favorable in all cases, and all patients were declared cured upon discharge.

Associated pathology We analyzed the associated pathology presented by patients, as shown in medical documents (Table 5). Of the 82 patients, 58 (70.7%) had a blood count at the time of admission, which revealed hemoglobin levels below 11g/dL. We found that the severity of methemoglobinemia was significantly higher among patients with hemoglobin levels below 11g/dl., (p=0.03). The concomitant presence of respiratory tract infections, cardiac pathology, or malnutrition was not significantly associated with a greater severity of methemoglobinemia. Eight patients (9.76%) experienced manifestations suggestive of an acute enterocolitis (fever, diarrhea, and vomiting). Of these in three cases, clinical and paraclinical changes of acute dehydration syndrome were obvious. The presence of dehydration syndrome was associated with a greater severity of methemoglobinemia (Mann-Whitney test, p<0.05).
A greater severity of methemoglobinemia was correlated with certain characteristics, including patients from rural areas, use of nitrates contaminated well water, age <1 year, presence of dehydration and hemoglobin values bellow 11g/dl, in a statistically significant manner. We analyzed the direct relationship between the severity of methemoglobinemia and the common presence of these parameters using multilinear regression. The results proved that the relationship between co-factors is complex and does not allow the establishment of a linear relationship between the severity of methemoglobinemia and a certain factor.
All patients received antidote treatment with methylene blue 5mg/mL solution intravenously, at a dose of 1mg/kg. The clinical status and methemoglobin value were analyzed during the first 24 hours post administration. In 60 cases (73.2%) cyanosis and the other symptoms disappeared in the first 60 minutes and no further administrations were required Of these 60 patients who received a single dose of antidote, 9 had methemoglobin values <20%, 31 had values ranging between 20 and 50%, and 20 had concentrations above 50% at the moment of diagnosis. Eighteen patients (21.9%) required two doses of methylene blue (1mg/kg), among whom 1 patient had an initial methemoglobin concentration <20%, 6 patients had values between 20% and 50%, and 11 patients had values >50%. Among the 4 patients (4.9%) requiring three doses of antidote, one patient had a methemoglobin concentration <20%, while the rest had concentrations above 50% before starting treatment.
Of the 22 patients who required additional doses of the antidote 20 (91%) had hemoglobin values bellow 11g/dl at the time of diagnosis. However, no significant association was reported between the need for additional doses of antidotes and the presence of hemoglobin values bellow 11g/dl. (Mann Whitney test, p=0.36). It was necessary to examine whether the lack of response to methylene blue administration is secondary to other causes. However, since this was a retrospective study, we did not have the values of glucose-6-phosphate dehydrogenase (G6PD) activity or Sul hemoglobin concentration. For all patients with acute dehydration syndrome, additional doses of antidote were required; in this situation, a statistically significant correlation (Mann-Whitney test p=0.009) was observed. All patients had a favorable outcome. In the analyzed group, there were no deaths and two patients required admission to the intensive care unit. The average duration of hospitalization was 3days; however, the majority of patients (51.2%) were discharged after 24h of staying.
Discussion
Acute toxic methemoglobinemia is a rare condition in pediatric toxicology, as evident in the present study, which revealed a prevalence of 1.58% of all intoxication cases in children. However, it can lead to severe functional anemia, especially in infants or in cases of associated pathologies. Early identification allows for quick initiation of antidote treatment with methylene blue, ensuring an excellent prognosis for patients.
Based on the data detailed above, the present study proposed a detailed analysis of cases of acute toxic methemoglobinemia from the point of view of etiology, clinical manifestations, associated factors (age, associated pathology), and their influence on the clinical form of the disease, as well as the outcome under treatment. The limitations of this analysis include difficulty in quantifying the extent to which each factor is independent of the severity of methemoglobinemia. the small sample size that hindered the predictive model, the difficulty associated with the evaluation nitrate concentrations in the wells of the patients and bias created by the type of admissions in an emergency hospital like the one we were referring to a large number of chemicals or drugs have been reported in literature as capable of producing oxidation of hemoglobin with the accumulation of methemoglobin. However, in this study, we identified only five categories of toxic substances inducing acute methemoglobinemia in children. Most of the cases in this study - 69 (84.1%), were secondary to exposure to nitrates contaminated well water used to prepare food in rural areas.
In a report published in 2009 by the National Institute of Public Health of Romania within the National Programme for Monitoring the Triggering Factors in the Life and Work Environment, 89 cases of toxic methemoglobinemia have been reported as a source of food contamination [5]. In the group of patients analyzed in the present study consisting exclusively of children, this source was not identified. In all cases, anamnesis referring the living conditions , using the risk assessment score for well water contamination with nitrates and nitrites [2]. indicated that the water used in the preparation of tea or milk was the source of the disease.
In 12 patients, the drug-induced cause of acute methemoglobinemia was identified. Of these, 10 patients (12.2%) were children with severe burns of at least II degree on extended body surfaces, who experienced episodes of acute methemoglobinemia during hospitalization. Analyzing the substances with the oxidant potential to which the children were exposed, we found that the common element was the repeated topical application of silver sulfadiazine on extended skin surfaces.
Silver sulfadiazine is an antibiotic of the sulfonamide class and exerts antibacterial and antifungal effects through the simultaneous action of silver ions and sulfadiazine. Its application on extensive skin areas and/or with significant destruction of superficial layers, such as burns, favors systemic action, leading to adverse effects similar to those of sulfonamides, including oxidation of hemoglobin with the formation of methemoglobin.
Another drug identified in our study as an inducer of methemoglobinemia was dapsone (dianinodiphenyl sulfone), a sulfone-class compound, considered in literature as one of the most common causes of toxic methemoglobinemia [6,7]. Dapsone is used in immuno depressed patients for the treatment of infection with Pneumocystis jiroveci or Toxoplasma gondii, in the treatment of leprosy, malaria, or some dermatological diseases, such as bullous pemphigoid and dermatitis herpetiformis.
In our study, dapsone was identified in a 4-year-old boy who was undergoing treatment for bullous pemphigoid. Clinical manifestations occurred at a methemoglobin concentration of 6.9%, which is consistent with the findings of other studies [8,9], confirming that therapeutic doses may trigger suggestive symptomatology at serum methemoglobin levels < 10%. Combination with other drugs with oxidant effects or concomitant presence of other diseases, such as heart, respiratory disease, and anemia, are considered to be promoting factors [9,10]. In the analyzed case, we only identified anemia with a hemoglobin level of 9.7 g/dL.
Another drug-induced cause of acute toxic methemoglobinemia was pentaerythritol tetranitrate.an organic nitrate that can induce methemoglobinemia in case of accidental or voluntary overdose. Methomoglobinemia occurs especially in cases of accidental or voluntary overdoses, with limited cases of methemoglobinemia occurring because of therapeutic doses.
In the analyzed group, we identified a female patient aged 2 years who presented with acute toxic methemoglobinemia due to accidental acute poisoning with pentaerythritol tetranitrate, with a methemoglobin concentration of 41.9%.
In one case, volatile substance were identified as etiological agents of methemoglobinemia. It was about a teenager aged 16 years, institutionalized, chronic consumer of substances of abuse, who presented with suggestive clinical manifestations: generalized cyanosis not responsive to oxygen administration, dyspnea, and tachycardia about an hour after smoking cigarettes soaked in a liquid street substance named “Liquid Gold”
Volatile abuse substances are another category with a well-documented role among the causes of toxic methemoglobinemia [11]. Volatile nitrites (amyl nitrite or isobutyl nitrite) are esters of nitric acid, which cause an intense vasodilating effect, with a rapid but short onset at the level of the central nervous system [12]. Although there are legislative regulations regarding the possession or sale of volatile nitrites in Romania as in the other European countries, these substances are available under various names: “Liquid Gold”, “Rush”, “Pig Black”, and are used both for inhalation and application in classical cigarettes. Most often, users of volatile substances are male adolescents, with poor socio-economic backgrounds, from disorganized families, and have low educational qualifications [13].
Although exposure to substances with oxidant potential may cause the accumulation of toxic concentrations of methemoglobin regardless of age, in the pediatric population, cases involving infants present the highest risk [14,15] due to functional particularities. The main peculiarity is the predominance of fetal hemoglobin, especially up to the age of 3 months, which can be oxidized much more easily than in adult form, which becomes predominant after 18 months of age. At this age, cytochrome b5 methemoglobin reductase activity is reduced [16], and gastric pH is less acidic than in older children or adults, thus facilitating bacterial proliferation, which plays an important role in converting nitrates in food into nitrites [17,18]. The analysis of the group of patients provided consistent results; the age subcategory of under 1 year being the best represented with 49 infants (59,7%) ,and the median age in the studied group was of 6 months.
Clinical manifestations are similar regardless of the incriminated oxidant substance and depend primarily on the concentrations of methemoglobin. In the vast majority of cases, levels below 10% are well tolerated, and patients are asymptomatic [15,19]. Cyanosis is the main clinical manifestation and is an indispensable clinical element for positive diagnosis along with an increase in methemoglobin values, as evidenced by literature. Most previous studies have shown that cyanosis becomes obvious at methemoglobin concentrations of more than 10%. However, in our study, the lowest value at which cyanosis was noted was 6.6%. As methemoglobin concentrations increase, the clinical picture is supplemented with changes secondary to hypoxia: cerebral symptoms (agitation, restlessness, irritability, headache and subsequently drowsiness, obnubilation, seizures, or coma), cardiac symptoms (tachyarrhythmias, circulatory failure), or respiratory symptoms (dyspnea, tachypnea, respiratory failure). Values above 70% are generally associated with an increased risk of death. In our study, the severity of clinical manifestations varied with the concentration of methemoglobin, but cases with values above 70% (72%–86% in our study) had a favorable outcome and no death was reported. There were 4 cases with methemoglobin values above 70%. All of them were infants and in addition to cyanosis presented tachycardia dyspnea and neurologic symptoms (drowsiness or agitation) Of these, 3 required three administrations of methylene blue and 1 required two doses.
In case of association with other pathologies that cause cell hypoxia (respiratory, cardiac, hematological conditions), the symptomatology is more severe at reduced concentrations of methemoglobin [15,20]. The analyzed group included a large number of patients with such associated pathologies, identifying a significant correlation between the presence of hemoglobin levels below 11g/dl, and the severity of clinical manifestations in patients with methemoglobinemia. Another situation encountered in the study, which has been significantly associated with a higher severity of the clinical picture of methemoglobinemia, was acute dehydration syndrome in patients with acute enterocolitis, most likely secondary to associated metabolic acidosis.
Exposure to methemoglobin zing substances can be life-threatening, so that the antidote treatment with methylene blue can be administered as soon as possible. Methylene blue is a thiazine coloring agent, which in the presence of NADPH methemoglobin reductase and NADPH is reduced to an intermediate metabolite, methylene blue leuco, capable of donating an electron to methemoglobin to reduce it to oxyhemoglobin.
The indication to initiate antidote treatment is symptomatic methemoglobinemia, since there are situations where the concentration of methemoglobin does not correlate with the severity of clinical manifestations [21]. The dose is 1-2mg/kg, administered intravenously over 3-5min, and repeated, if necessary, when the symptomatology does not cease after 60 min, up to a maximum dose of 7 mg/kg [9,15].
Although extremely effective in a vast majority of situations, the administration of methylene blue is not without risk. Digestive side effects (nausea and vomiting), electrocardiographic abnormalities (inversion of T wave, flattened R waves), profuse sweating, dyspnea, retrosternal pain, and oral dysesthesia usually occur at doses above 7 mg/kg [9]. It can also cause a paradoxical increase in methemoglobin through its oxidant effect, especially in patients with glucose-6 phosphate dehydrogenase deficiency. In the study group, methylene blue administration was limited to a single dose in most situations, 73.2% (60 patients), and the majority (51 of 60) had methemoglobin concentrations over 20%. In 22 cases, it was necessary to administer one or two additional doses, most of them [16] having hemoglobin levels lower than 11g/dl. Even that there were not possible to establish a significant association between the need to supplement the initial dose and the presence of hemoglobin levels lower than 11g/dl. The total dose of methylene blue used in the treatment of patients in this group did not exceed 3 mg/kg, and no adverse effects of the treatment were reported.
Conclusion
Acute toxic methemoglobinemia is mainly observed in children up to 5 years of age. This age group represents 94% of all cases in our study, of which the majority belong to the subcategory of 0-1 year. In countries as Romania where the rural infrastructure is less developed the main cause of acute toxic methemoglobinemia in children is the exposure to nitrates contaminated well water used for food preparation. Silver sulfadiazine cause acute toxic methemoglobinemia when applied to extensive areas, repeatedly and/or under the condition of significant destruction of the surface layers of the skin. Methemoglobinemia secondary to exposure to volatile substances, such as amyl nitrile or isobutyl nitrile, used as new psychoactive substances, is a newly described condition in recent years and must remain under the attention of specialists.
Although the relationship of co-factors is complex and is difficult to quantify to what extent the severity of the clinical picture is in linear correlation to a certain factor. We believe that the age under one year, hemoglobin values lower than 11g/dl, and acute dehydration syndrome are factors that can aggravate clinical symptomatology and outcome in acute toxic methemoglobinemia.
Methylene blue is the treatment of choice for acute toxic methemoglobinemia in children, and its administration is effective in a single dose of 1mg/kg in 73.2% of cases without adverse reactions.
To Know more about Open Access Journal of Toxicology
Click here: https://juniperpublishers.com/index.php




No comments:
Post a Comment