Annals of Reviews and Research - Juniper Publishers
Abstract
Cyperus rotundus also known as ‘Nutgrass’ have been used in Ayurveda and in Traditional Chinese medicinal practices for its multiple medicinal property. Since obesity is major factor for the increase in the metabolic syndrome related complication. We have used Cyperus rotundus methanolic extract in mice along with the High fat diet. As compared to only high fat diet group, Cyperus rotundus administered high fat diet showed significant weight reduction along with reduced triglycerides and cholesterol level. Cyperus rotundus also acted on inflammation, reducing the TNF alpha level in mice fed with Cyperus rotundus along with the high fat diet. This was also connected to the level of reduction in the mRNA expression of COX-2 in the visceral fat of the mice.
Keywords: Cyperus rotundus; Anti-obesity; Ayurveda; Herbal plant; TNF-alpha, Anti-inflammatory; Hypolipidemic
Abbreviation:HFD: High fat diet; CRR: Cyperus rotundus roots; TNF: Tumor necrosis factor; COX: Cyclo-oxygenase; BW: Body weight
Introduction
Obesity is a public health problem in both developed and underdeveloped countries. It might be owing to the complex interaction of genetic, nutritional, behavioural, and environmental variables that contribute to morbidity and death. In other words, it is produced by a higher calorie intake than expenditure. Obesity is commonly characterised as an abnormal or excessive quantity of fat build-up, to the point where it has a negative impact on health. Obesity pathogenesis promotes the development of metabolic illnesses (e.g., Type 2 diabetes, fatty liver disease, hypertension, hyperlipidaemia, osteoarthritis, cancer, low-grade inflammation, sleep apnea, and cardiovascular disease) [1]. Obesity's complicated pathophysiology necessitates the discovery and development of novel medications and solutions for its prevention or treatment. Because present medications are failing to provide long-term treatments, natural items, particularly medicinal herbs, are thought to be viable antiobesity agents. Different natural product combinations work synergistically on multiple molecular targets via diverse ways, either boosting weight reduction or avoiding weight gain [2]. Medicinal plants' mechanisms for combating obesity include inhibition of lipid hydrolysing and metabolising enzymes, disruption and modification of adipogenic factors, and appetite suppressants [3].
Anti-obesity mechanism of Cyperus rotundus
Cyperus rotundus has numerous medicinal properties which include anti-cariogenic, anti-viral activity, anti-Candida, cytotoxic effect, inhibition of Brain Na+ K+ ATPase, neuroprotective effect, anti-emetic, anti-arthritic, hypotensive, cytoprotective, cardioprotective, anti-hyperlipidaemic, anti-malarial, anti-allergic, hepatoprotective, anti-histamine, ovicidal and larvicidal effect, gastroprotective, anti-helmintic, analgesic, anti-ulcer anti-platelet, anti-obesity, anti-convulsant, anti-microbial, anti-hyperglycaemic, anti-diarrheal, anti-inflammatory, anti-pyretic, wound healing, anti-oxidant property [4]. Cyperus rotundus is a perennial herb native to India, used to treat various ailments such as diarrhoea, diabetes, pyrosis, inflammation, malaria, bowel disorder, cancer, hypertension, and allergy . The major organic chemicals isolated from various parts of the Cyperus species include quinonoid pigments, sesquiterpenoids, flavonoids and stilbene derivatives [5]. Several monoterpenoids, amino acids and fatty acids are also reported in the plant. The ethyl acetate extract of dried, pulverized rhizomes of C. rotundus was reported to contain Scirpusin A, Scirpusin B & Piceatannol as major compounds [6]. The chloroform/methyl alcohol extraction of C. rotundus yielded novel enantiomeric and meso-stilbene trimers, such as Cyperusphenol A as well as other stilbenoids (Cyperusphenol C & D, Scirpusins A & B, Piceid and Luteolin [7].
Materials and Methods
Extraction: The Roots of Cyperus rotundus L. were purchased from local market and its authenticity was done by Prof. NK Dubey and the voucher has been preserved in Centre of Advanced study in botany Herbarium and in our department under voucher no. Cypera.2021/4 and YBT/MC/2021/4 respectively as dry material. It was dried in sun light and coarse powder was made. This powder was filled in a thimble of blotting paper and put in Soxhlet apparatus for extraction. For the preparation of total methanol extract CRR (Cyperus rotundus roots), the powder CRR were extracted with methanol separately in Soxhlet extractor for 30 hrs. For extract preparation, weighed the amount of sample was refluxed in round bottom flask by two hours on water bath. The solvent was filtered out and the process was repeated two times. The solvent from all these steps were collected and distilled in vacuum distillation plant. The solvent free extract was prepared by drying the solvent on water bath and by desiccation in the vacuum desiccation till constant of the weight. Animals: Animals were procured from the central animal facility of our own Institute. They were inbred animals of Swiss albino mice. The animal tests were carried out in accordance with the ethical clearance committee of Banaras Hindu University's Institute of Medical Sciences. Varanasi, India. The study in this research work were approved vide letter no. Dean/2021/CAEC/2567 dated 07.03.2021. Anesthesia: Experiments were carried out on healthy adult male albino mice of the Swiss albino strain weighing between 15-20 gm. and kept in our animal facility for 7 days, on animal food and water ad libitum, for deworming and acclimatization in a controlled condition of room temperature, humidity (50 percent RH), and light (12 hr:12 hr light dark period).
Preparation of High fat diet
It contained lard (177.6 g/lit) Casein (200gm/lit) L-cysteine (3gm/lit), Corn starch (72.8gm) Maltodextrin(100gm/lit), Sucrose (172.8gm), Soyabin oil (25gm) Reagents: SGPT, SGOT <Glucose estimation were purchased from Accurex Biomedical Pvt. Ltd., Thane, India. Lipase substrate, 4-nitrophenyl butyrate, DPP-4 substrate and ABTS+ tablet was purchased from sigma -aldrich. Tnf-alpha Elisa kit was purchased from Elabscience, USA Nitroblue tetrazolium chloride, Metaphospheric acid (HPO3)n, Methiionine, Riboflavin were purchased from Hi-Media, Calcutta, Hemocord-D, a hemoglobin reagent was purchased for Coral Clinical system, Goa, India
Methods
To induce obesity twelve healthy albino mice of Swiss strain of inbreed colony (weighing 20-30 gm) were randomly divided in to 2 groups of 6 individual (n=6). Each of which was designated as control (C) was kept on normal diet and other was supplemented with high fat diet Above treatments were continued for 4 months. This group contained 6 mice.
For the effect of CRR on the HFD fed mice we divided the groups under 4 groups.
i. Standard Diet (Chow diet )
ii. High fat diet (45 %kcal)
iii. High fat diet (45 %kcal)+ 750mg/kg CRR Extract.
iv. High fat diet (45 %kcal)+ 1500mg/kg CRR Extract.
v. High fat diet (45 %kcal)+ 3000mg/kg CRR Extract.
Estimation of Body weight -Body weight was taken every 10th day in the duration of 60 days. The weight was taken 3 times and its average was considered.
Estimation of serum cholesterol-10 ul of serum was combined into 1 ml of working solution and incubated for 5 minutes at 370C or 10 minutes at room temperature (25-300C). After incubation, the absorbance of the assay mixture is measured at 510 nm in comparison to a blank. If not exposed to direct light, the finished colour remains consistent for 2 hours.
i. Calculation: Conc. (mg%) = Absorbance of sample/Absorbance of standard ×200
Determination of Serum glucose: 10 μl of serum was combined into 1 ml of working solution and incubated for 10 minutes. The finished colour will remain steady for at least 1 hour.
i. Calculation: Conc. (mg%) = Absorbance of sample/Absorbance of standard ×200
Oral Glucose Tolerance Test (OGTT): In overnight fasted mice, oral glucose tolerance tests (OGTTs) were performed. Following a minor tail incision, blood samples for glucose measurement were collected. The concentrations of glucose in the blood were measured using a glucometer (SD-Chek India) at 0, 15, 30, 45, and 60 minutes following an oral dose of glucose (2 g/kg body weight dissolved in 0.9 percent saline solution
Estimation of SOD activity: 1.25 ml of SOD buffer (pH 7.8), 600 l methionine, 150 l EDTA, 300 l NBT, 200 l riboflavin, and 500 l of properly diluted tissue homogenate were included in the 3 ml reaction mixture. The percentage inhibition was determined in relation to the control (without the enzyme source).
Estimation of catalase: 2.0 ml of diluted homogenate in 0.1M phosphate buffer was added to the reaction mixture (enzyme extract). To begin the reaction, 1.0 ml of 200 mM H2O2 was added. The reduction in OD per minute was measured against a blank (all reagents except enzyme extract) for 3 minutes at 240 nm at 15-second intervals.CAT activity was expressed as mg/ protein in tissue and was calculated as follows: U/mg protein = ΔA/min ×Total reaction mixture volume/Sample Volume × E × protein (mg/ml) X DF
Estimation of Lipid Peroxidation (LPO): 1.5 ml of 10% TCA solution was added to 100 l of tissue homogenate. After 10 minutes, spin at 5000 rpm for 10 minutes. The supernatant was separated and combined with 1.5 mL of TBA. The tubes were placed in a boiling water bath for 30 minutes to finish the reaction before being cooled with tap water. The absorbance of the sample at 535nm in comparison to distilled water.
i. Calculation: MDA (nmol) = Concentration of Standard TEP (nm) × Experimental OD /Absorbance of standard TEP
Estimation of TNF alpha: Mouse TNF- alpha was estimated through ELISA kit ,the assay procedure for it was followed as per the Manufacturer protocol
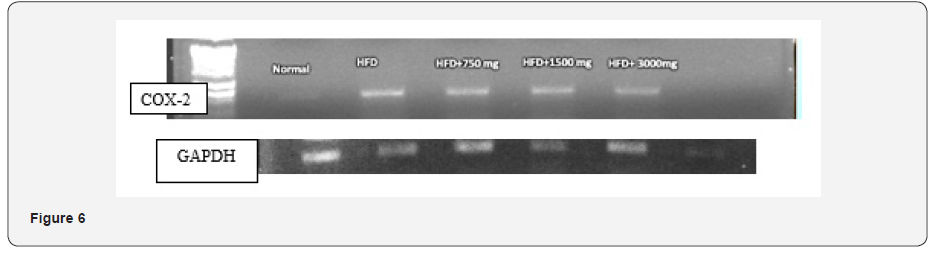
Estimation of COX-2: Reverse transcriptase PCR of visceral fat tissue was used. RNA isolation was done with the Trizol reagent. The CDNA was prepared with the GAPDH Primer Forward Primer 5’CACGGCAAGTTCAATGGCACA 3’and reverse primer GAATTGTGAGGGAGAG TGCTC as constitutive gene and COX-2 forward primer 5’ TGGGTGTGAAGGGAAATAAGG 3’, reverse primer 5’CATCATATTTGAGCCTTGGGG 3’.
Results
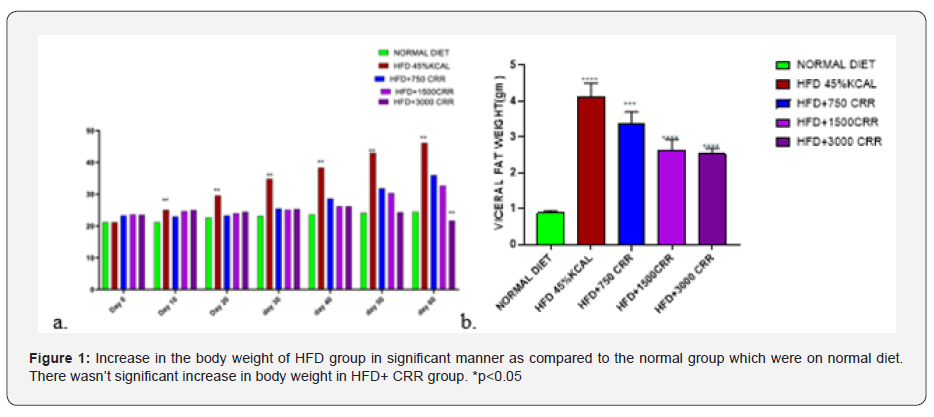
Estimation of Body weight- In the 60 days study, there were significant differences in body weight among the normal diet, HFD groups and CRR groups. The increase in the body weight was observed after 20 days in the significant manner with the p<0.05. The HFD group gained the weight in the course of 60days. In contrast the CRR fed HFD mice showed stagnant increase in body weight (Figure 1).
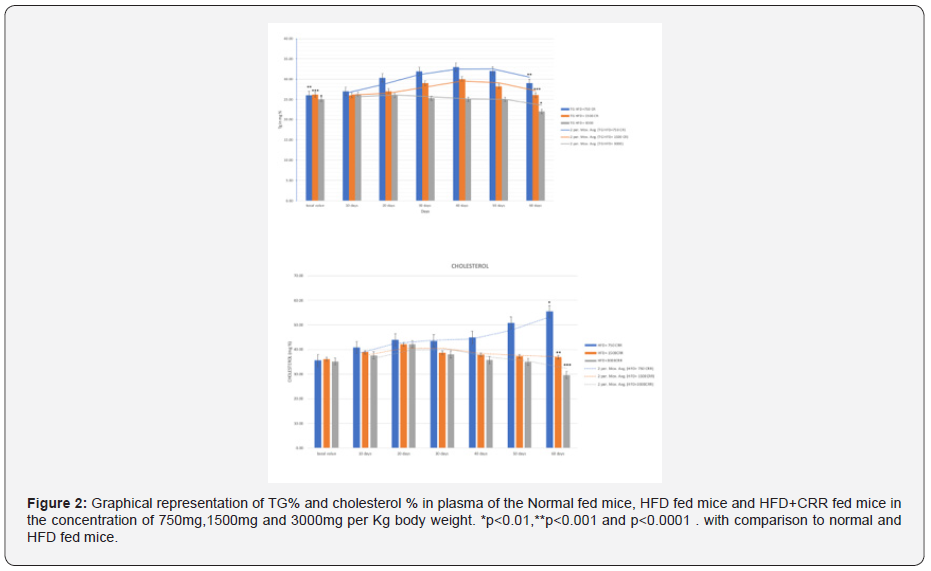
Estimation of Lipid profile
The administration of HFD and Normal diet in mice shown the increment in both TG and Cholesterol percentage during the 60 days. In HFD group the TG percentage of the mice on 60th day was 60.85 ±0.49 which was increased by 1.09 times as compared to normal fed mice of the 60th day having its value of 60th day of 55.66 ± 1.17. Effect of HFD shown the significant increase in Triglyceride level. In Normal diet fed group the cholesterol percentage of the mice was 50.83±0.45 on 60th day and HFD group shown the 55.51%+ 0.51 cholesterol percentage on 60th day. Cholesterol level was increased by 1.09 times in the HFD group as compared to Normal diet fed mice The administration of HFD along with the CRR in mice shown the increment in both TG and Cholesterol percentage during the 60 days, but it was significantly reduced as compared to HFD diet. In HFD group the TG percentage of the mice on 60th day was 60.85 ±0.49 which was increased by 1.09 times as compared to normal fed mice of the 60th day having its value of 60th day of 55.66 ± 1.17. Effect of HFD shown the significant increase in Triglyceride level. Cholesterol percentage during the 60 days was also significantly increased in HFD group as compared to Normal fed mice. In Normal diet fed group the cholesterol percentage of the mice was 50.83±0.45 on 60th day and HFD group shown the 55.51%+ 0.51 cholesterol percentage on ^0th day. Cholesterol level was increased by 1.09 times in the HFD group as compared to Normal diet fed mice (Figure 2).
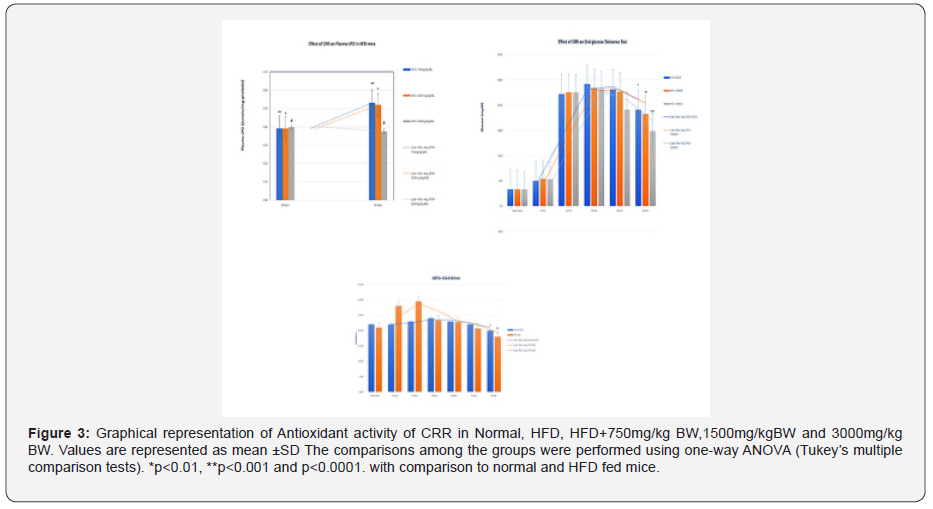
Effect of CRR on HFD fed mice on antioxidant enzyme activities in blood hemolysate:
The SOD and catalase activity were decreased in HFD fed mice group in gradient manner. Significant reduction in SOD level was observed from 30 days onwards to 60 days i.e from (0.419 ± 0.017) to (0.233 ± 0.014) (p<0.005). SOD level from 30 days to 60 days was downregulated by 1.8 times in HFD group. In contrast, Normal diet fed mice (NFD) group shown no significant changes in SOD. Comparing the NFD group of 60 days to HFD group of 60 days there was downregulation of SOD by 2.0 times (0.458± 0.02 to 0.233± 0.014). In catalase, the NDF group there was no significant changes in catalase from 10 to 60 days. In HFD, significant change was observed from its basal value . The changes were statistically significant (both P<0.05). The decrease in catalase was observed on 10th to 60th day in gradual manner .In comparison of HFD to NFD ,the mice on 60th day shown the catalase value of (0.388 ± 0.08) as compared to NFD mice (1.277 ± 0.029) .In the estimation of Plasma LPO , there was no significant change in NFD group but in the HFD group there was significant increase in plasma LPO as compared to its basal value and also with the 30 days (p<0.001). At 60 days in HFD group the mean levels of TBARS reached to 0.579 nmol/mg protein which was significant higher compared with their baseline and 60 days NFD mice (p<0.001) The ABTS+ scavenging potential in HFD group was also decreased in this period when compared with NFD group. It was significantly (p<0.001) declined on the 60th day. Thus, it could be suggested that changes in activity of SOD, catalase and ABTS+ scavenging potential are some of the sensitive parameters for early detection of HFD feeding related metabolic changes (Figure 3).
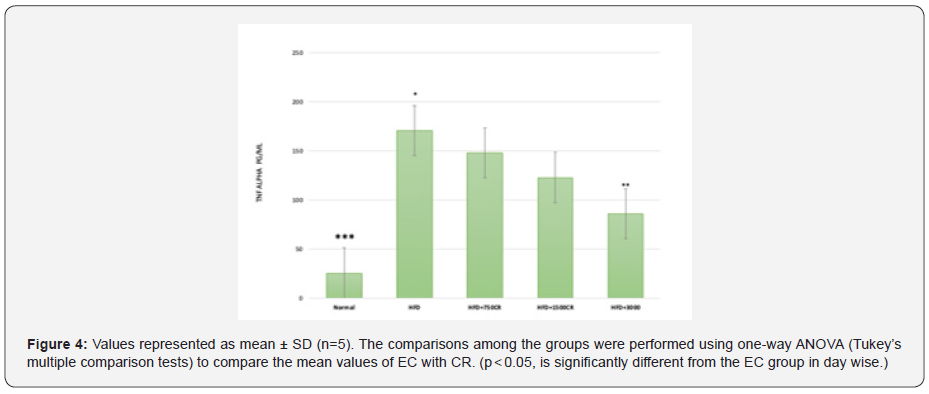
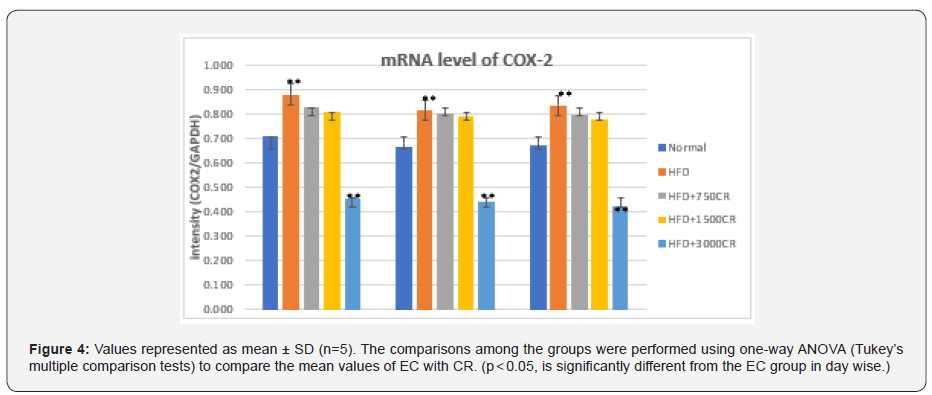
Estimation of TNF alpha- HFD fed mice (Figure 4)
Estimation of COX-2
The COX-2 mRNA expression in visceral fat was significantly higher in high fat diet fed mice group on the 60th day. In comparison to HFD, the CRR fed in HFD mice i.e HFD+3000mg/kg BW showed the significant reduction in the level of mRNA expression of COX-2. Group 3- HFD+ 750mg/kg BW CRR, and HFD+1500mg/kg BW CRR also reduced the COX-2 expression as compared to HFD (Figure 5 & 6).
Conclusion
Since The state of obesity can trigger the occurrence of oxidative stress conditions due to prooxidant and antioxidant imbalance in the body so that it will form Reactive Oxygen Species (ROS) [8]. Obesity occurs excessive lipogenesis and inhibition of lipolysis [9]. Lipogenesis is stimulated by a diet high in carbohydrates. The major antioxidant enzymes that neutralise Reactive Oxygen Species (ROS) are superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx). SOD catalyses superoxide dismutase into H2O2 and oxygen, whereas CAT and GPx neutralize H2O2. Finally, high ROS production and decreased antioxidant capacity lead to a variety of disorders, including endothelial dysfunction, characterised by decreased endothelial vasodilation and systemic RBC bioavailability [10]. In the state of obesity can occur chronic inflammatory conditions of low levels with progressive infiltration of immune cells in obese adipose tissue [11]. Cytokines secreted by immune cells and adipokines of adipose tissue promote tissue inflammation [12]. The administration of CRR in 750mg/kg BW ,1500mg/kg BW and 3000mg/Kg BW in HFD fed mice for 60 days showed the reduction in all biochemical parameters. Dose of 3000mg/kg BW of CRR in HFD fed mice significantly reduced the triglycerides and cholesterol level. As CRR have earlier shown its property as antioxidant, in the blood hemolysate it showed the quenching of free radicle which are being produced in the presence of HFD [13]. This quenching could be the enhancement in antioxidant mechanism such as SOD ,Catalase and ABTS+. In our antioxidant test, gradual increase in the SOD, Catalase and ABTS+ defence mechanism was improved significantly. Similarly, lipid peroxidation (LPO) was reduced in CRR fed mice of HFD. This indicates that CRR in these HFD fed mice, managed to maintain the equilibrium of lipid metabolism. In the visceral fat study, as CRR have earlier been reported as TNF-alpha inhibitory [14], this could explain the inhibition of VEGF, as TNF-alpha action on adipocytes can directly alter lipid metabolism through inhibition of FFA uptake and lipogenesis and stimulation of FFA release via lipolysis [15]. In this way, adipose tissue-derived TNF-α can contribute to the development of dyslipidaemia and resultant metabolic complications [16]. In this experiment we have seen that COX-2 level have also been reduced in increasing order of CRR concertation in the HFD fed mice. Henceforth it can be concluded that CRR shows its activity as antioxidant which in results causes downregulation of inflammatory cytokines such as TNF alpha and COX-2. Due to this, histology of visceral fat fad of CRR fed mice showed the smaller size of adipocytes as compared to HFD. All these cascading results of CRR in the obese mice, could be utilised as anti-obesity plant.
To Know more about Annals of Reviews and Research
Click here: https://juniperpublishers.com/index.php





Hi, this is Arunprakash. I like the way the article is explained. The content is very good. If any of the engineering students who are looking for a Artificial Neural Network Projects I found this site and they are providing the best service to the engineering students regarding the projects Artificial Neural Network Projects
ReplyDeleteThis blog is very helpful and informative for this particular topic. I appreciate your effort that has been taken to write this blog for us. Best Adenoidectomy Surgery in Hyderabad
ReplyDeleteThis blog is very helpful and informative for this particular topic. I appreciate your effort that has been taken to write this blog for us. Functional Endoscopic Sinus Surgery In Hyderabad
ReplyDelete