Cancer Therapy & Oncology - Juniper Publishers
Abstract
Introduction: One of the most effective chemotherapeutic agents is 5-fluorouracil (5-FU). The aim of this study was to investigate its effects on bone marrow cell proliferation and autophagy in adult female C3H mice.
Methods: C3H mice and bone marrow cells were exposed to 5-FU and its toxicity was evaluated mediating microscopic analysis, colonies formation, pH variations, apoptosis and autophagy.
Results: After ablating bone marrow with 5-FU treatment, a rapid regeneration occurred during the subsequent 10 days. This was achieved by hematopoietic stem cells positive to autophagy/PI dye. Hence, the restoration of chemically depleted bone marrow was apparently initiated by cells normally resident in that tissue. We examined the recovery of colony formation by evaluating BFU-E, CFU-GM and CFU-GEMM. At 10 days post-administration of 5-FU, a full recovery of the formerly depopulated bone marrow was observed, with similar cell populations in treated and untreated mice. Bone marrow recovery is intrinsic to the hematopoietic progenitor cell compartment. Recovery was dependent on the autophagic cells that survived 5-FU ablation.
Conclusion: A compartment of primitive stem cells exists in adult female C3H mice and suggest that these cells may have constituted the surviving population responsible for reconstituting the bone marrow after a single 5-FU treatment.
Keywords: 5-fluorouracil; Bone marrow; Autophagy; Apoptosis; Hematopoiesis
Introduction
One of the most effective chemotherapy agents is 5-fluorouracil (5-FU), which competes with uracil during the synthesis of ribonucleic acid (RNA) and interferes with deoxyribo nucleic acid (DNA) formation by blocking thymine production [1], preventing cell division. This selective effect on rapidly proliferating cells is the basis for its carcinostatic potency [2-5].
Cell death can occur through of different mechanisms; however, apoptosis, autophagy and necrosis are the most studied. Autophagy is an important cellular clearance process by which a cell internally delivers damaged organelles and macromolecules to lysosomes for degradation [6-8]. The recent focus on understanding the intricate interplay between apoptosis, necrosis and autophagy has revealed several important regulators. Anticancer chemotherapy drugs challenge hematopoietic tissues to regenerate. Chemotherapy-induced deficits in hematopoietic stem or stromal cell function have been described, but the mechanisms mediating hematopoietic recovery remain unclear [9]. The aim of the present study was to investigate the effects of 5-FU on bone marrow cell populations, as well as the capacity of this tissue to recover from such treatment through cell proliferation and autophagy. We quantified the cellular recovery with colony formation analysis. By assessing of the uptake of acridine orange, we could identify the bone marrow cells that may have been responsible for the regeneration of this tissue after a 5-FU challenge. We herein confirm that administration of 5-FU causes substantial ablation of the recipient marrow, induces tissue destruction and reduces hematopoietic regeneration, showing that the induction of autophagy is a survival response against 5-FU genotoxic insult.
Material and Methods
Animals
Adult female C3H mice, 8-12 weeks old and weighing 23 ± 2 g, were employed (n=8). Animals were exposed to a single dose of saline (control) or 100 mg/kg 5-FU by IP injection. After 10 days of exposure, animals were sacrificed, and paired tissue samples were snap frozen in liquid nitrogen and placed in a -80°C freezer or fixed in 10% buffered formalin for 24 h and then processed into a paraffin embedded block. A suspension of bone marrow cells was prepared as follows: femurs were first rinsed with phosphate-buffered saline (PBS), both ends of femurs were cut and bone marrow was flushed by 29G x ½ needle and collected in a tube. Cell suspension was centrifuged at 1200 rpm x 6 min and cells were washed twice with PBS. A homogeneous suspension was prepared in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and a mix of antibiotic-antimycotic. All animal care and operative procedures were approved by the Ethics Committee.
Microscopic studies
Bones were fixed in 3.7% formaldehyde and embedded in paraffin. Slices (5 μm) were stained with hematoxylin and eosin and evaluated blindly by using bright-field microscopy. Studies were performed under a Leica IV/05 inverted microscope equipped with a Hitachi HV-F22 color camera and Empix Imaging (Northern Eclipse, Mississauga, ON, CA) software for analysis and image processing.
Immersion decalcification
Femurs were harvested after CO2 euthanasia, and surrounding soft tissue was trimmed down. The specimens were fixed with 10% neutral buffered formalin and immersed in 10% ethylenediamine tetraacetic acid sodium salt (EDTA-2Na), 4% paraformaldehyde/phosphate buffered saline (PFA/PBS) adjusted to pH 7.3 with Trizma® base (Sigma, St. Louis, MO, USA) at 4°C.
Immunostaining and flow cytometry analysis of erythroid differentiation
Single cell suspensions were prepared from femur bone marrow. Cells were washed in Iscove’s Modified Dulbecco’s medium (IMDM), counted, and resuspended in PBS plus 2% FBS staining medium (SM) at 5 × 106 cells per milliliter. Then, 1 x 106 cells were incubated with mAbs for 30 minutes, washed twice in SM, and resuspended in 0.2 mL of SM for analysis by means of a Becton Dickinson FACSCalibur (Mountain View, CA, USA) flow cytometer. The following primary antibodies were used: anti-CD71-PE, anti-Terr119-FITC (1:100 dilution; Cedarlane Laboratories, ON, CA).
In vitro effect of 5-FU on bone marrow and evaluation of progenitor cells
In a 96-well plate (n=3 wells/group) were seeded 2 × 105 bone marrow cells from femurs of normal mice. The cells were treated with 100 pg/ml 5-FU (or untreated) in serum-free medium for 24 h. The medium was replaced by fresh medium containing 50 ng/mL of stem cell factor (SCF) and the cells were incubated for 72 h more. At the end of this assay, methylcellulose cultures were set up at 104 cells/well with 50 ng/ml of SCF, 10 ng/ml of IL-3, 10 ng/ml of IL-6 and 3 U/ml of EpO. Cell mixtures were plated in 35-mm suspension culture dishes (Nunc, Roskilde, Denmark) and incubated at 37°C. After 10 days, colonies (>100 cells) were counted in each of three triplicate plates. Colonies were assessed microscopically.
Intracellular pH monitoring and quantification of bone marrow cells
Variations in pH were monitored on a SPEX model CM1T11I dual excitation spectrofluorometer, by using the fluorescent cytoplasmic pH indicator, 2',7'-bis(2-Carboxyethyl)-5(6)-carboxyfluorescein (BCECF) (Molecular Probes, Eugene, OR, USA) [10]. After a 30-min loading period with 10 mM BCECF, cells were monitored at 37°C in a thermostatic cuvette under constant agitation. The ratio of the pH-sensitive fluorescence (excitation wavelength: 500 nm) and pH-insensitive fluorescence (excitation wavelength: 450 nm) allows for the calculation of pH. The optical density (OD) values were obtained by using the membrane peroxidase 1.2phenylendiamindihydrochloride system. To determine the membrane peroxidase, cells were serially diluted in media and placed directly on a standard microtiter and then processed.
Cell apoptosis and autophagy analysis
The cell apoptotic rate was determined by the TUNEL assay (In Situ Cell Death Detection Kit, TMR red; Roche Applied Science, Basel, Switzerland). Bone marrow cells (5 × 105) were seeded onto a 24-well cell culture plate and treated with 5-FU (100 pg/ml) or non-treated. After 48 h, cells were washed with PBS and fixed with 4% paraformaldehyde in PBS (pH 7.4) for 1 h, and then resuspended in 0.1% sodium citrate containing 0.1% Triton X-100 for 2 minutes on ice. The cells were treated with TUNEL reaction mixture containing terminal deoxynucleotidyl transferase (TdT) and fluorescein-dUTP and then incubated at 37°C in a humidified atmosphere in the dark for 1 h. After washing with PBS, the cells were analyzed by using a flow cytometer (FACSCalibur; BD Biosciences). Data analysis was carried out with Cell Quest software. The red fluorescence indicates apoptotic cells.
Detection of acidic intracellular vesicles
The acidic vesicles (i.e. lysosomes, autophagolysosomes) were visualized by supravital staining with acridine orange (1 μM; Sigma-Aldrich) for 15 min at 37°C. Bone marrow cells treated with 5-FU (100 pg/ml) and non-treated were analyzed under an inverted fluorescent microscope (Leica Microsystems DMIL, Wetzlar, Germany) using a Leica Microsystems DFC320 camera and Leica Application Suite software (version 2.8.1). Alternatively, cells stained with acridine orange were examined on a FACSCalibur flow cytometer using Cell Quest Pro software. Accumulation of acidic vesicles was quantified as a red/green fluorescence ratio (mean FL3/FL1). The analysis was performed after 48 h of 5-FU treatment.
Statistics
Data were analyzed for statistical significance with the Student’s t test. Differences were considered significant at p<0.05.
Results
Bone marrow cell populations in the red series were lower in 5-FU-treated versus untreated mice
Bone marrow cells could be divided into four populations, based on forward and right-angle light scattering. Erythroid cells, at different maturational stages, were found in three of these four bone marrow subpopulations. The sequentially correlated expression of two cell surface markers, CD71 and Terr 119, enabled the study of erythroid maturation from the colony forming cell to the mature erythrocyte. CD71 was expressed on the earliest identifiable erythroid cells and was progressively lost as the cells matured. Its expression began at the E-BFU stage and disappeared at the late reticulocyte stage. Unlike CD71, Terr 119 increased and remained at a constant quantity at the mature erythrocyte stage [11,12]. In the 5-FU-treated mouse bone marrow, all three cell populations diminished, suggesting a failure in cell differentiation and proliferation (Figure 1A).
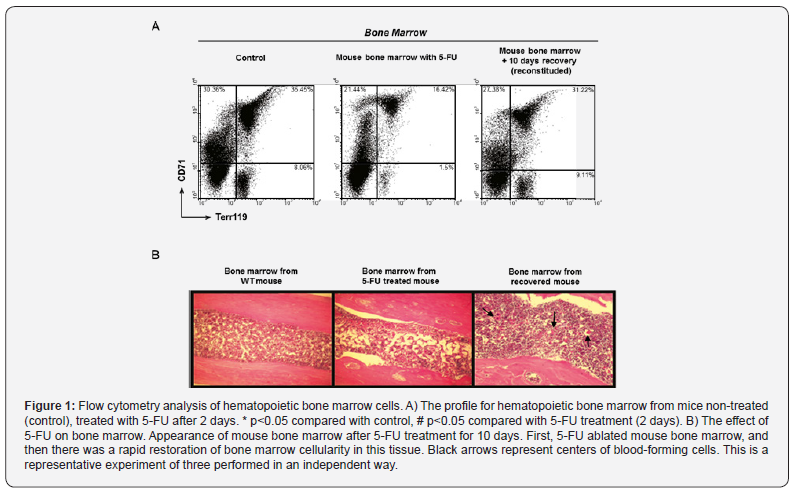
The relation between the cells that survived by autophagy and blood destruction and regeneration
In mice, 5-FU generally produces a depression of bone marrow activity in the long bones. During the early periods (1-6 days), the change in bone marrow is robust and either focal or peripheral. After 7 to 10 days, the replacement of new bone marrow cells is complete or nearly so (Figures 1A & 1B). In figure 1B, numerous centers of blood-forming cells are found in bone marrow from recovered mouse (black arrows).
Normal bone marrow activity recovered after 5-FU treatment
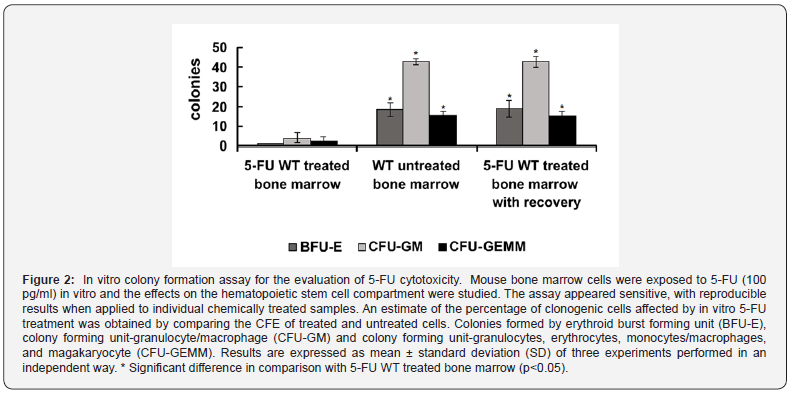
Mice herein submitted to 5-FU treatment recovered 10 days after exposure to the challenge. We evaluated the BFU-E, CFU-GM) and CFU-GEMM in bone marrow cultures 10 days after application of 5-FU, observing a similar proportion in all colonies in the bone marrow of 5-FU-treated bone marrow with recovery and untreated mice, compared with 5-FU WT treated bone marrow (Figure 2), corresponding to the first days of repair in the treated mice. The BFU-E gives rise to the erythroid colony-forming unit (CFU-E), which when stimulated produces erythroid colonies and amplifies the differentiation processes in response to erythropoietic stress (e.g 5-FU) [10,13].
Cytochemical and functional characteristics of bone marrow cells are maintained in dividing cells
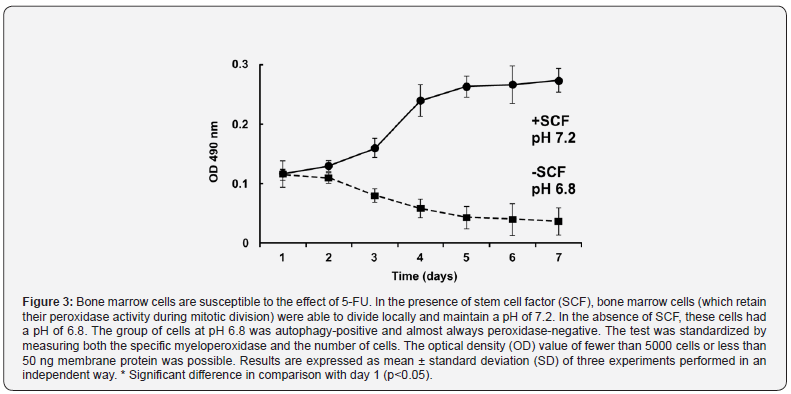
The results indicated that bone marrow cells, which retain their peroxidase activity during mitotic division, can divide locally in the presence of SCF around day 4 and maintain a pH of 7.2, but without SCF have a pH of 6.8. Cells without growth factor were almost always peroxidase-negative and autophagy positive. The test was standardized by measuring both specific myeloperoxidase and the number of cells (Figure 3).
The action of 5-FU on live cells cultured in vitro
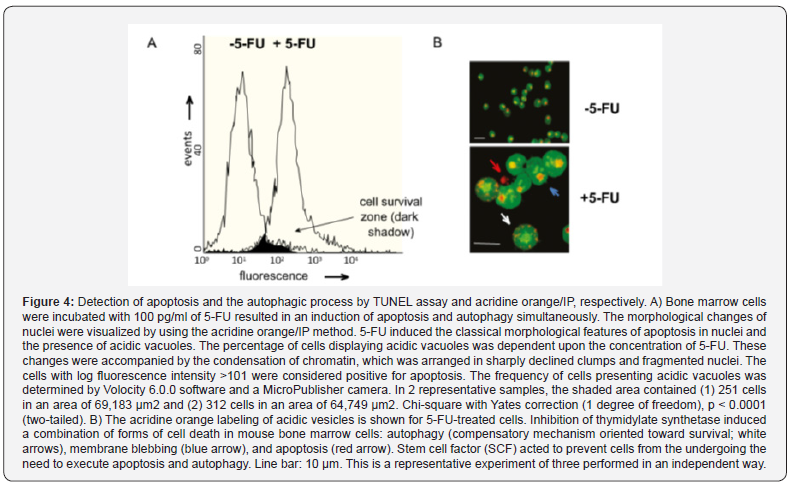
The cytotoxic effects of 100 pg/ml of 5-FU were so overwhelming that the subsequent cell death was almost complete (Figure 4A). The value calculated to quantify the toxicity of 5-FU for bone marrow cells is the fraction expressed as the lethal effect divided by the control. The shaded area (cell survival zone) is the intersection of the lethal effect of 5-FU and the control. When utilizing concentrations below 25 pg/ml, no cytotoxic effects of 5-FU were observed on bone marrow cells (data not shown). Figure 4B shows the promotion of autophagy by 5-FU and that SCF inhibits starvation-induced autophagy based on the change from the reddish-orange-colored spots of the acridine orange-stained vesicles to a yellowish color (indicative of interference with the acidification step required for completion of autophagy).
Discussion
There are numerous reports on changes in bone marrow following 5-FU treatment. Studies of bone marrow done in our lab, conducted with the analysis of many normal mice, suggest that this is the most rational method for exploring compensatory changes because numerous centers of blood-forming cells are normally found. In this sense, it is known that 5-FU-induced anemia in mice follows a gradual downward course for 5 days, in which the decrease in hemoglobin is relatively more marked than that of red blood cells. Likewise, an equally gradual repair causes the red blood cell count and hemoglobin content to approach normal after 10 days or more. Hence, we have attempted to establish whether the hyperplasia in bone marrow after 5-FU treatment is compensatory: (1) mainly through the overactivity of red blood cell formation, (2) by intensified activity in regard to white blood cell formation, or (3) as an orderly reproduction of new marrow with normal activities that form all cells that arise therein. Our study of femur bone marrow in normal mice has revealed that the basic elements usually detected are undifferentiated cells and myelocytes, which lie between fat cells and seem to be in no way related to blood channels. In these centers, cells of the connective tissue reticulum are evident. Some other patterns commonly accompany these basic elements: a peripheral accumulation of cells in which the leukocyte series predominate, a mantle of cells in which the erythrocytic series are most evident, and an indiscriminate mingling of red and white blood cells. Moreover, at times there are groups composed only of white or of red blood cells without myelocytic centers.
We have found that the search for the groupings around myelocytic centers greatly facilitates the study of complex marrow pictures, readily leading to a decision as to whether leucoblast or erythroblast activity predominates. Such a view is supported by the fact that all hyperplastic bone marrow contains large amounts of altered blood pigment that is found mainly in large phagocytic cells. Although this is sometimes free, it mostly occurs in large phagocytic cells. The activity of these phagocytic cells presumably cleanses the microenvironment to stimulate the other functions of the bone marrow, to wit the erythrogenic and leucogenic functions, which eventually cause a replacement of the dead cells by a very cellular red marrow. In adults, bone hematopoietic cells are responsible for the lifelong production of all blood cells. Hematopoietic stem cells have the capacity to self-renew and give rise to the entirety of mature blood.
In the present study, autophagic hematopoietic cells markedly alleviated the destructive effects of 5-FU following in vivo exposure. We present evidence that ablation of mouse bone marrow by 5-FU was followed by rapid restoration. A small proportion of cells positive to vital colorants like acridine orange is responsible for reestablishing the bone marrow. Moreover, bone marrow recovery is intrinsic to the hematopoietic progenitor cell compartment and is dependent on the autophagic cells that survive the effects of 5-FU. The current results agree with prior reports showing bone marrow recovery [2-5,14,15].
Mouse bone marrow cells were exposed to 5-FU in vivo and the effects on the hematopoietic stem cell compartment were studied. The drug was highly toxic to bone marrow cells, including BFU-E, CFU-GM and CFU-GEMM populations. The number of colonies of these distinct cell types was similar when comparing normal bone marrow cells and 5-FU-treated cells at 10 days post-injection. Furthermore, cell colonies derived from 5-FU-treated bone marrow decreased as much as 10-fold during the first 10 days post-treatment.
Several studies have shown that the stem cell compartment is heterogeneous regarding its self-replicative capacity and developmental potential [16]; accordingly, there may be an age structure for this compartment [17]. It has been proposed that cells having a short mitotic history are more likely to self-replicate and cells with a longer mitotic history are more likely to differentiate. Based on this hypothesis, 5-FU may be toxic to the older stem cells and selectively spare the more primitive subpopulation. The current results demonstrate that a stem cell compartment at the level of primitive cells exists in these mice, and that it may possibly reconstitute the bone marrow after 5-FU treatment.
Stem cells within the bone marrow exist in a quiescent state or are instructed to differentiate and mobilize to circulation following specific signals [18]. Bone marrow ablation by 5-FU induces recruitment of c-Kit stem cell progenitors [19]. To define the basis for the faculty of the hematopoietic function, autophagic cells were studied. These cells translocate to a permissible vascular niche favoring differentiation and reconstitution of the stem/progenitor cell pool.
Examination of the hematopoietic status in mice treated with a high dose (over 100 mg/Kg mouse body weight) of 5-FU evidenced that the damage to bone marrow hematopoiesis was extreme. Indeed, hematopoiesis was effectively eradicated in mice given this dose, leading to death from bone marrow failure. The time of death and hematopoietic status of these animals are compatible with the onset of hypoplastic bone marrow failure that causes pancytopenia and death.
Ten days after applying the mild dose of 5-FU treatment, on the other hand, a repopulation of myeloid, erythroid, lymphoid and megakaryocytic cell lineages was observed in mouse bone marrow. These data indicate that after mild 5-FU treatment, hematopoietic stem cells can provide the basis of recovery to normal proliferation. According to the present data, mice can recover because autophagy is involved in the phagocytosis of dead cells. Clearance of the apoptotic cells by phagocytes plays an important role in the maintenance of tissue homeostasis as well as the promotion of immunological tolerance and the anti-inflammatory response [20,21]; however, the contribution of autophagic destruction of apoptotic cells by macrophages or other cells is not well defined.
In mice, cell differentiation by hematopoietic stem cells after mild 5-FU treatment results in hematopoietic recovery and decreased mortality [22,23]. Hematopoietic stem cells have the capacity to self-renew and give rise to the entirety of the mature blood throughout the lifespan of an organism. Doan et al. [24] have demonstrated instances of an ex vivo expansion of isolated and cultured murine bone marrow (BM) CD34(-)ckit(+)Sca1(+)Lineage(-) (CD34(-)KSL) hematopoietic stem cells. These characteristics and instrumentation were used herein to observe primitive cells at various developmental stages in conjunction with autophagy. Autophagic clearance of the insoluble aggregates mitigates the toxic effects of 5-FU on the surviving cells.
Autophagy and cell death are intimately linked. Not only do both processes employ the same molecular machinery, but some studies also suggests that autophagy and apoptosis can occur simultaneously [25-27]. Several researchers have shown that apoptosis induction is often associated with increased autophagy [28]. It has also been demonstrated that apoptotic cell death is enhanced by the inhibition of autophagy [29]. Hence, there is an interplay between these two important cellular events. The simultaneous activation of both phenomena has been detected not only in experimental settings but also in vivo under physiological and pathological conditions [30,31]. The current results established the role of autophagic cell death in the rapid recovery of mice after the application of 5-FU. It is generally thought that when chemotherapy promotes autophagy, this has a cytoprotective function [32].
Conclusion
Our results show that a compartment of primitive stem
cells exists in adult female C3H mice and suggest that these stem cells
may have constituted the surviving population responsible for
reconstituting the bone marrow after a single 5-FU treatment (Figure 5).
We have posed a possible mechanism whereby stem cells improve
phagocytotic function and serve as a direct mediator of increased
clearance. The present results on hematopoietic bone marrow cell
populations support qualitative clinical evidence of the anti-tumor
potency resulting from therapy with 5-FU.
To Know more about Cancer Therapy & Oncology
Click here: https://juniperpublishers.com/index.php





No comments:
Post a Comment