Biomedical Engineering & Biosciences - Juniper Publishers
Abstract
Introduction: Female infertility is a condition that is currently treated through the maximization of existing reserves; a necessity due to the fact that a true reversal of the processes leading to infertility is not yet technologically possible. This experiment examined the ability of mesenchymal stem cells to differentiate into primordial germ cells (PGC) when cultured onto the placental scaffold.
Methods: To produce the scaffolds, the cotelydons were collected and decellularized by umbilical vessel SDS perfusion. Adipose derived cells were isolated based on rapid adherence to the plastic.
Results: The isolated cells displayed the markers CD90 and 105, while lacked CD34 and CD45. When seeded onto the scaffold, the cells successfully differentiated into PGC like cells, displaying typical PGCs markers, including STELLA, OCT4, DAZL and VASA.
Discussion: These materials were chosen for their low cost and wide availability. The data in this study show the promising potential of cell-scaffold complex to support the development of female tissue engineering-based regenerative medicine therapies.
Keywords: Placenta; Female Infertility; Ovarian Regeneration; Tissue Engineering; Adipose- Derived Cells
Introduction
Assisted reproductive techniques (ART) are currently accepted as the most effective way to overcome infertility in humans [1]. In animals, however, the objective of ART is to reduce generational intervals and to propagate genetic material among breeding animal populations. The most-used ARTs are artificial insemination, embryo transfer, in vitro embryo production, and cloning. That being said, ART has some limitations, including its requirement for healthy individuals in which to apply the technology [2]. Female infertility affects 13% of women worldwide, a level of prevalence that is not limited to humans. Systematic infertility is also a pressing concern in agriculture, where it currently poses a threat to global livestock supply chains, particularly that of the dairy industry [3]. The importance of this problem in both bovine species and humans allows for a doubly beneficial translational investigation. The bovine species represent an excellent model for investigation of this topic due to the similarity of their anatomies, physiologies, and lifespans to those of humans [4]. Cows possess particularly strong similarities to humans in regard to their reproductive system, which extend to the areas of physiology, folliculogenesis, oocyte development [5], the oocyte selection processes, and age-associated endocrinal events, among others [6].
Oocyte production disorders are some of the most common causes of fertility disruptions [7], and ovarian failure has been associated with autoimmune disease, toxic effects of chemotherapy, and radiotherapy, all leading to progressive ovarian depletion [8]. There are several treatment options for infertility, and the approach used depends on the root cause of the disorder. Options include hormonal regulation, ovarian tissue cryopreservation, autologous or allogenic transplantation, and immature or mature oocyte or embryo cryopreservation [8,9]. The appropriateness of autologous graft depends on the patient’s condition, as the graft can cause secondary donor site injury and possesses an inherent risk of reintroduction of carcinogenic material in oncology patients [9,10]. Allogenic transplantation, however, is not always the preferred choice, as it runs the risk of provoking an immune response and even tissue rejection [9]. Moreover, there are several key parameters that are important for the success of the transplantation, such as graft size, transplantation site, vascularization, and oxygen levels in the transplanted area [11], that must all be taken into account when evaluating the appropriateness of a transplantation-based treatment. Additionally, the post-operative functionality of the graft depends on the condition of the transplanted follicle reserve and its activation. Once the follicles are active, the reserve of primordial follicles decreases, which, in turn, decreases the lifespan of the ovarian graft [11].
In order to overcome the risks of immune reaction by allogenic transplantation and reintroduction of undesirable material by autologous transplantation, efforts have been made to create ovarian follicles in vitro. There are several 3D models that attempt to mimic in vivo oocyte conditions in vitro, including agarose gels for in vitro oocyte maturation [12], fibronectin [13-15], fibrin [16], alginate beads [17] and alginate droplets [18]. Interestingly, when immature follicles were isolated from prepubescent mice for in vitro culture in alginate droplets and subsequent fertilization and embryo transfer, it resulted in fertile offspring [18]. The use of fibrin grafts has been shown to restore cyclicity in an infertile mouse model [16]. Although these results may encourage the use of cryopreserved oocytes in ART, some important practical challenges remain. For example, the management of an acceptable hormone treatment and oocyte collection timeframe for patients with cancer in order to avoid delays that could compromise the survival of the healthy tissue [8]. In this context, tissue engineering strategies have become clinically relevant to treat female infertility. The success of this approach depends on several factors that must be taken in consideration, including the choices of scaffold and cell source. Mainly, three types of scaffolds have been used: (1) naturally derived polymers, (2) synthetic polymer scaffolds, and (3) decellularized tissues. This third type is advantageous due to the fact that it provides a native extracellular matrix (ECM) that possesses natural biological composition, microarchitecture, and vasculature [19,20].
In addition, the removal of cellular contents circumvents immune reaction by the host [20]. The ideal scaffold should have the capacity to slowly degrade, enabling remodelling by the ECM proteins secreted by the cells, which is a quality possessed by biological scaffolds [9]. Currently, the most-used decellularized tissue matrices for female reproductive regeneration are: porcine small intestinal submucosa [21-26] and amniotic membrane [27] for vaginal reconstruction, peritoneum for cervical aplasia [28], and ovary to restore ovarian function [29]. For recellularization in tissue engineering approaches, numerous cell types can be used, including tissue specific stem cells, mesenchymal stem cells, and pluripotent stem cells [10]. Regarding ovarian tissue engineering, the major problem of using tissue specific stem cells is the chance of reintroducing tumor cells [9,10]. It was shown that grafts obtained from bovine decellularized ovaries seeded with primary ovarian cells could initiate puberty in ovariectomized mice [29], which represents an interesting opportunity for translational medicine, although it fails to address the objective of developing an autologous system in which the oocytes would originate from the host organism. The use of pluripotent cells is clinically limited as well, especially due to their propensity for tumor formation and genetic instability [30]. Thus, mesenchymal stem cells represent a promising cell source to be used in the recellularization process, not only because they does not form tumors when injected in vivo but also because they are easy to obtain and differentiate, exert immunomodulatory effects, and are able to modulate angiogenesis, cell proliferation, and apoptosis [31,32]. Taken together, the aim of this study is to investigate the potential of decellularized placenta to be recellularized with adipose tissue-derived cells for further primordial germ cell differentiation.
Methods
Scaffold production
The placenta was frozen, immediately after collection. The scaffolds were produced by isolation of the cotyledons and subsequential perfusion for 2 days with 0.01% sodium dodecyl sulfate (SDS) followed by 2 days of perfusion with 0.1% SDS. The decellularization process and evaluation of its accuracy was performed at Dr. Miglino’s lab and then donated for the re-cellularization and differentiation experiments [33].
Cell derivation and culture
Adipose tissue samples were collected from six female Bos taurus indicus. Prior to tissue harvest, the area to be incised was washed, shaved, and anaesthetized by a local epidural injection of 2% lidocaine chlorhydrate. The tissue was then collected from the base of the tail. On average, 1 g of tissue was used for enzymatic digestion, which was performed with collagenase type I for 3 h in agitation at 37 °C. After this period, the digested tissue was washed in expansion culture medium, which is composed of Dulbecco's Modified Eagle Medium (DMEM, Invitrogen, Grand Island, USA) supplemented with 10% fetal bovine serum (FBS, Cripion, Brazil) and 2.5mg/mL of amikacin (NovaFarma, Brazil). After centrifugation, the pellet was transferred into a T25 culture flask (Corning, New York, USA), and the medium was changed three hours after the beginning of the culture, a protocol that has been previously described for the selection of a mesenchymal stem cell population at the first passage [34,35].
Cell differentiation
To verify the mesenchymal nature of the isolated cells, a differentiation test was performed in three biological and technical replicates. During the 21 days of the differentiation procedure, the medium was changed every 4 days, and commercial differentiation medium kits were used for osteogenic and chondrogenic differentiation (StemPro; Thermo Fisher Scientific). In order to evaluate lineage differentiation, a cytochemical staining was performed following the manufacturer's instructions. Von Kossa stain was used for osteogenic differentation and Alcian blue stain for chondrogenic differentiation.
Cell seeding onto the scaffold
In order to rule out any microorganism proliferation in the scaffolds, they were sterilized with UV light and cultured in expansion medium for 5 days. The scaffold was placed into a well of a 96-well plate for subsequent cell seeding, in a density of 1.0 × 104 cells per well, in biological triplicates. During the period of 21 days, the cell-scaffold complexes were cultured with either expansion or differentiation medium in a 2D rocker system that completed 24 cycles per minute to aid homogenization of the cell-scaffold complex. In order to have a 2D environment control group, the cells were cultured in the absence of the scaffold expansion medium for 21 days. For primordial germ cell induction, the cells were cultured in a previously reported medium that was originally used to maintain ovarian cells in vitro, but also was able to successfully differentiate MSC into PGC-like [34,35], which was DMEM supplemented with 10% FBS, 0.1 mmol/L β‐mercaptoethanol (Sigma‐Aldrich), 10 ng/mL LIF (Santa Cruz Biotechnology, Santa Cruz, CA, USA), 20 μg/mL transferrin (Sigma‐Aldrich), 5 μg/mL insulin (Sigma‐Aldrich), 60 μmol/L putrescine (Sigma‐Aldrich), 10 ng/mL EGF (mouse epidermal growth factor; Invitrogen), 40 ng/mL human GDNF (glial cell line‐derived neurotrophic factor; Invitrogen), 1 ng/mL human bFGF (basic fibroblast growth factor; Invitrogen) and 2.5mg/mL of amikacin.
Scanning Electron Microscopy (SEM)
In order to evaluate cell interaction with the scaffold, biological triplicates of the cell-scaffold complexes were observed using a scanning electron microscope (Zeiss EVO MA-10). After 21 days of culture, the complexes were fixed in Karnovsky solution for 48 h and dehydrated in ethanol. Prior to observation with the microscope, the complexes were dried with critical point equipment and metalized in gold.
Histochemical staining
In order to visualize cell adherence to the scaffold, a histochemical staining assay was performed on the biological triplicates of cell-scaffold complexes using Hematoxylin-eosin staining (HE). After 21 days of culture, the cell-scaffold complexes were fixed in 4% buffered paraformaldehyde (PFA) for 48 h, dehydrated in ethanol, diaphonized in xylene, embedded in paraffin, sectioned by a microtome, and placed onto glass slides for visualization by light microscopy (Eclipse 80i, Nikon).
Quantitative reverse-transcription assay
Gene expression quantification was evaluated by quantitative real time polymerase chain reaction (qRT‐PCR). This assay was performed in the six isolated cell lines and in biological triplicate of cell-scaffold complexes. The first step in this process was to extract RNA from the cells using the RNeasy Mini Kit (Qiagen, Hilden, Germany), for subsequent treatment with DNase I (Qiagen, Hilden, Germany) to prevent genomic contamination of the samples. 500 ng of total RNA was used for reverse transcription using the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany). The primers were designed using the NCBI primer design tool, and gene expression quantification was performed (7500, Applied Biosystem). The geometric mean of two housekeeping genes was used to obtain the relative expression of each studied gene. The statistical analyses were performed using a T-test in GraphPad Prism. P < .05 was considered to be statistically significant.
Immunohistochemical staining
Cells at the first passage, cultured in the absence of the scaffold, were fixed with 4% PFA for 48h. The cell-scaffold complex was also fixed with 4% PFA for 48h after 21 days, whether in the group cultured with expansion medium or differentiation medium. The cells and the complexes were incubated at room temperature for 1h with a blocking solution with 2% bovine serum albumin/PBS, followed by overnight incubation with the primary antibodies. After three washing steps, the cells and the complexes were incubated with secondary antibody for 1 h at room temperature, followed by a 10 minute incubation with 4′,6-diamidino-2-phenylindole (DAPI) for nuclear staining in blue. The negative control was performed by the incubation of the cells or cell-scaffold complex with the secondary antibody only. The cells and cell-scaffold complexes were observed in a confocal microscope (FV1000, Olympus).
Results
Scaffold production
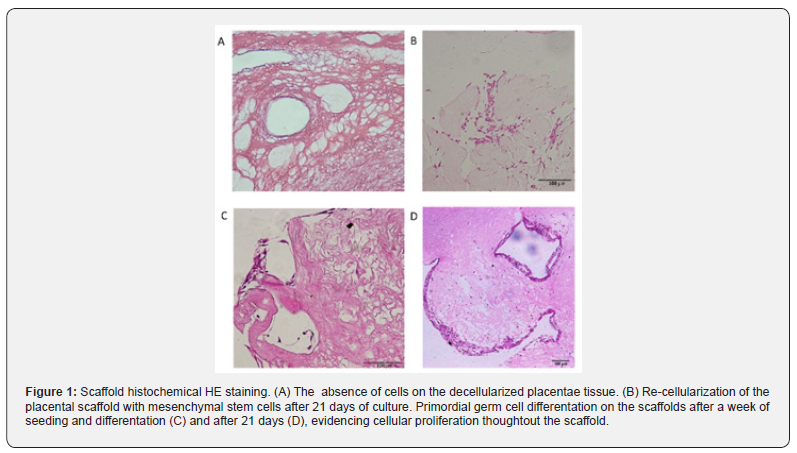
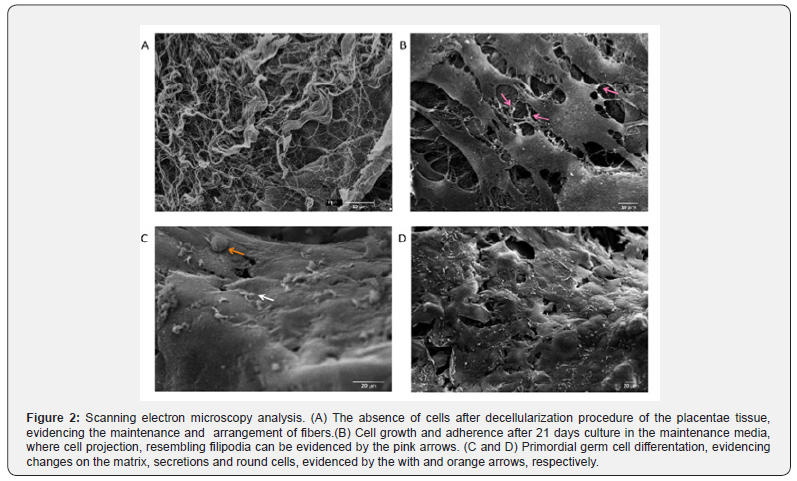
The placental scaffolds were successfully produced and validated [33]. It is possible to observe the absence of cells after the decellularization process in Figure 1A with HE stain and, by SEM analysis, the fiber arrangement can be observed in detail (Figure 2A).
Cellular characterization
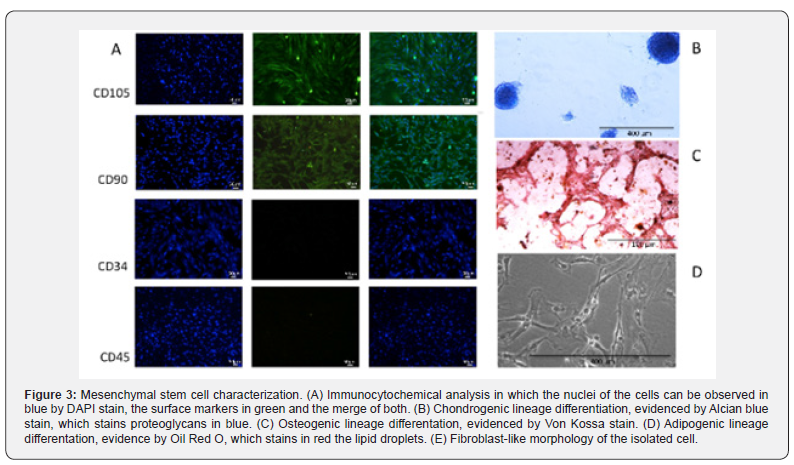
Cell selection based on rapid adherence selected cells with fibroblast-like morphology, which can be observed in Figure 3. The cells grew in a homogeneous monolayer for more than 14 passages. Additionally, the isolated cells displayed typical mesenchymal cell surface antigens, such as CD90, CD105 and CD73, while they did not display hematopoietic markers CD34 and CD45, which can be observed in Figure 3A. Moreover, the isolated cells were able to undergo differentiation into two mesenchymal lineages: chondrogenic (Figure 3B) and osteogenic (Figure 3C), in compliance with the MSC characteristic criteria suggested by the International Society of Cellular Therapy [36].
Cell-scaffold interactions
Next, the MSC were cultured with the placental scaffold for 21 days in a dynamic 2D rocker system. As can be observed in Figure 1B, the cells of the control group, which were cultured with expansion medium, adhered to the scaffold. Additionally, those cells established cell-cell communication, and the filopodia may be responsible for cell adherence to the scaffold and monolayer growth (Figure 2B).
Cell differentiation on the scaffold
Lastly, the potential of MSC to differentiate into PGC-like when seeded onto placental scaffolds was evaluated. In Figure 1C, HE staining reveals that the cells adhered to the scaffold one week after seeding, however, more cells are observed 21 days after differentiation (Figure 1D). After this period, the cells seeded onto the placental scaffolds displayed PGC related markers, including DAZL, VASA, STELLA and OCT4 (Figure 4). The SEM analysis shows cell morphology differentiation and cell secretion (Figure 2C & 2D) when comparing the differentiated group with the control (Figure 2B).
Discussion
The bovine species was chosen as the experimental model in this study due to its advantages over other translational models, such as rodent, which include more similar organ sizes and physiology than small animal models [4,37]. Concerning specifically the reproductive physiology, the bovine species is more closely related to humans in regard to folliculogenesis, oocyte development, endocrinal events and age related-events [5,6]. In the field of reproduction, mesenchymal stem cells have been reported to be a promising option for infertility treatment. This is owed to a number of beneficial qualities that they possess, including their wide differentiation potential [34,35] and paracrine effects, which modulate angiogenesis, cell proliferation, and apoptosis, as well as the fact that their isolation does not involve ethical concerns [31,32]. Previous studies have tested the effects of adipose derived stem cells on the regeneration of ovarian tissue, and it was observed that mesenchymal stem cells have the ability to prevent and rescue granulosa cells from apoptosis [38,39], as well as increase the number of ovarian follicles [40-46] and the pregnancy rate [46].
The ability of mesenchymal stem cells to differentiate into germ-like cells under appropriate stimuli and to be transplanted into ovarian tissue has been previously reported [see review, 1]. However, it is known that 3D culture systems mimic in vivo conditions more closely than 2D systems, allowing for rates of cell growth, proliferation, and differentiation that are similar to those observed in vivo [47]. The ECM of placental scaffolds possesses a number of important similarities to that the in situ ovarian niche, for example, its richness in laminins and collagens [33,48-50]. Moreover, placental tissue is a very promising scaffold source due to its rich vasculature, abundant availability, and maintenance of fibers after decellularization [33]. Herein, we provide evidence that bovine mesenchymal stem cells can successfully differentiate into primordial germ cells when seeded onto placental scaffolds and cultured with specialized medium for natural ovarian primordial cells. This is the first report to show the differentiation of mesenchymal stem cells into PGC like cells in a 3D culture.
There are several important issues that should be taken into consideration when attempting a transplantation, including vascularization, oxygen levels, and graft size [11]. Regarding these issues, placental scaffold is an advantageous choice of material due to its well-developed vasculature and ability to build anastomoses with the host tissue [51,52]. Moreover, regarding vascularization and oxygen levels, mesenchymal stem cells are the best option to be transplanted with the placental scaffolds, since they have angiogenic abilities and stimulate the development of vessels in the transplanted area [53,54]. Another important topic is that of immune response to the transplanted graft, for which placental scaffolds seeded with MSC once again have a great advantage, specifically because of the low immunogenicity of MSC [54-56] and the fact that the decellularized tissue has low potential to cause inflammation and immune rejection once cell contents and antigens are removed [57]. However, an important concern of decellularized tissues is the remaining chemicals after the process that could possibly be harmful [57]. In this study, we provide evidence that the cells were able to grow, adhere, and proliferate when seeded onto the decellularized placenta, which demonstrated that, if there were remaining chemicals, they were not detrimental to the re-cellularization process. Now, specifically regarding ovarian graft transplantation, the primary concern is the functionality of the transplanted graft, since follicle activation leads to a decrease of the primordial follicle reserve, consequently decreasing the lifespan of the graft [11]. Placental scaffold with MSC could be an option to overcome the deleterious effects of this process, as MSC are known to increase blood vessel concentration, which, in turn, could stimulate the formation of new follicles and, incrementally over time, their activation, seeing as the ability of MSC to increase ovarian follicle numbers and improve folliculogenesis has previously been demonstrated [40-42].
PGC differentiation is a complex process. After embryo gastrulation, Oct-4 expression is confined to the germline, and is expressed again during folliculogenesis, coincidently with the entry into meiosis [58]. Due to the fact that Oct-4 is expressed by other cell types, including totipotent, pluripotent, and some somatic stem cells, it remains challenging to use it as a determining marker for germ cell specification [59]. Before colonizing the gonads, Stella may have an important role in germ cell development and differentiation [60]. The population of cells that express Stella seems to be a restricted lineage of germ cells, which continues the expression during migration to the gonadal ridge [61]. During the end migratory phase and arrival at the gonads, the PGC express VASA, which seems to be required for the maintenance of germ cell functionality [59,62,63]. After gonadal colonization, PGC are characterized by the expression of VASA, DAZL, and NANOS3 [for review see 63]. Dazl is a cytoplasmic protein involved in primordial follicle formation [64]. In the present experiment, when the cell-scaffold complexes were cultured with ovarian cell expansion medium for 21 days, it was possible to verify the expression of specific PGC markers, including Oct4, Stella, Vasa, and Dazl, demonstrating the potential of those cells to differentiate into primordial germ cells after the transplantation of the MSC-scaffold complex into the ovary. In order to capitalize on the potential revealed by this experiment for advances in human reproductive medicine, further experiments must be conducted with the aim of specifying and clarifying certain important details. Chief among these is the in vivo evaluation of transplanted MSC-scaffold complexes, which will provide data regarding the ability of the complexes to restore ovarian function. Another important matter to be investigated is whether a transplant conducted with MSC that have already differentiated into PGC-like would evoke a more efficient outcome than undifferentiated MSC with regard to restoring ovarian function. These proposed experiments would greatly further the potential of these techniques and clear the way for novel regenerative therapies to treat female infertility.
Conclusion
Here, we provide evidence of the ability of MSC to maintain their mesenchymal nature when seeded onto placental scaffolds as well as to differentiate into primordial germ cells when cultured in an ovarian cell maintenance medium. However, future experiments must to be performed in order to test the behaviour of the cell-scaffold complex in vivo.
To Know more about Biomedical Engineering & Biosciences
Click here: https://juniperpublishers.com/index.php




No comments:
Post a Comment