Open Access Journal of Toxicology - Juniper Publishers
Abstract
α-Lipoic acid (LA) is a strong antioxidant compound widely used in oxidative stress-related clinical conditions. LA exists in two enantiomeric forms, R-(+)-lipoic acid (R-LA) and S-(-)-lipoic acid (S-LA). Nowadays LA is synthetized mainly by a chemical processes that produce the racemic mixture, but only the R-LA is the biological active form. A lot of different formulation of LA are available on the market and the majority of them contains the racemic mixture, and few data are available on the impact of the inactive enantiomer on the pharmacokinetic profile. However, it is known that the S-LA is somewhat impacting the PK of the products. This study aims to evaluate the pharmacokinetic profile of a new patented oral liquid formulation (Liponax® sol) containing 300 mg of R-LA in 10 healthy volunteers. Blood samples were collected up to 180 minutes after the consumption of 300 mg R-LA in fasting subjects. Plasma concentrations of R-LA were determined by ultra-high performance liquid chromatographic coupled with mass spectrometer (UHPLC-MS). R-LA was rapidly absorbed showing a Cmax value higher than the commonly recognized lipoic acid therapeutic effect activation threshold and a high AUC if compared to other published data where 600 mg racemic LA tablets were administered.
Keywords: R- α-lipoic acid; UPLC-MS; Pharmacokinetics; Blood samples, Liponax sol
Abbreviations: LA: Lipoic Acid; UHPLC-MS: Ultra-High-Performance Liquid Chromatographic Coupled with Mass Spectrometer; LOD: Limits of Detection; LOQ: Limit of Quantification; SIR: Single Ion Recording; SD: Standard Deviation; AUC: Area Under the Concentration–Time Curve; NA: Naproxen
Introduction
α-lipoic acid (LA), also known as thioctic acid or 1,2-dithiolol-3-pentanoic acid, is a small amphiphilic organosulfur molecule produced by plants, animals, and humans [1]. Due to the chiral carbon in C6 position, LA exists as two enantiomeric forms: R-(+) lipoic acid (R-LA) and S-(-) lipoic acid (S-LA), of which R-LA is the naturally exsisting compound. In fact, R-LA is covalently bound to lysine residues as lypoyllysine and occurs in many vegetable (i.e. spinach, broccoli, peas and tomatoes) and animal foods (i.e. kidney, heart, and liver), in very low amounts, ranging from about 1 mg/g to 22 mg/g and 2 mg/g to13 mg/g, respectively [2]. R-LA is an essential cofactor for mitochondrial enzymes involved in energy production and cellular metabolism (i.e. pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, and branched chain α-ketoacid dehydrogenase complex) [3]. In humans, due to the extensive tissue diffusion capacity, LA crosses the blood-brain barrier and is localized at cerebral cortex level [4]. The most important feature of this molecule is its powerful antioxidant activity. Along with its reduced form (dihydro-lipoic acid, DHLA), it is a potent redox couple with a redox potential of -320 mV [5] which makes it able to directly regenerate other natural antioxidants, such as oxidized form of glutathione (GSSG/GSH couple with a redox potential of -140 mV [6]) and vitamin C, and indirectly vitamin E. Moreover, DHLA can be recycled from LA [7,8] and for this reason the LA/DHLA couple has been called the “universal antioxidant”. R-LA can exert its antioxidant activity in both cellular membranes and cytosol, differently from other endogenous antioxidants, which exert their activity only in hydrophilic or hydrophobic environment. This molecule shows metal-chelating capacity [9,10] and the ability to scavenge hydroxyl radicals, hypochlorous acid, and single oxygen [11]. R-LA activates the insulin signaling pathway in insulin responsive tissues [12], stimulates glucose uptake by translocating and regulating the intrinsic activity of GLUT4 [13], participates in lipid metabolism [14,15], increases insulin sensitivity [16,17].
Although R-LA is the naturally occurring and most active form it is rarely used due to its intrinsic susceptibility to polymerize. Nowadays, LA is synthetized mainly by a chemical process that produce the racemic mixture (more stable) that is widely used as drug or food supplement ingredient [18,19].
A lot of different formulation of LA are available on the market and the majority of them contains the racemic mixture, and not much has been investigated on the impact of the inactive enantiomer on the pharmacokinetic profile. It is known that the S-LA is somewhat impacting the PK of the products due to its influence on the polymerization susceptibility of the R-LA few literature data underline the potential negative effects of the S-LA [19,20].
Administered intravenously, LA achieves the maximum plasma level leading to beneficial effects in the treatment of symptomatic diabetic polyneuropathy and other diabetic related conditions. The pioneering ALADIN study [21] shows that intravenous LA in diabetic patients with neuropathy causes a significant improvement in symptoms, such as burning, paraesthesia and pain, compared to placebo. On the other hand, LA is a food supplement available only for oral administration usually as tablets or capsules containing 600 mg racemic LA [22]. Unlike the intravenous administration, the bioavailability of LA tablets is lower through the oral route. In the past, the bioavailability of various solid formulation was investigated and it was established a low grade of LA absorption around 30% [23]. Indeed, phenomena such as reduced solubility in acidic environment and enzymatic degradation, which characterize its gastric and hepatic passage when administered orally, limit the potential of LA. Moreover, the oral administration involves various aspects that restrict the amount of LA absorbed, such as the disintegration of the solid formulations, the first pass effect in the liver, the inter-individual variability [10,23]. For this reason, various chemical interventions and formulations have been tested to achieve greater plasma bioavailability of LA even after oral administration and to ensure better therapeutic effects [18,24- 27]. To date, there is only one patented oral liquid formulation on the market containing 300 mg of the R-(+) enantiomer stabilized as Na salt in water and co-solvent [28]. This formulation has shown improved pharmacokinetic parameters in experimental animals when administered orally at 50mg/kg dose and in an experimental model of diabetes induced in Sprague Dawley rats it resulted to improve nerve conduction velocity, hyperglycaemia and hypertriglyceridemia [19]. Nowadays, an improved liquid formula, Liponax® sol, is available as food supplement and its biological activity, tolerability and safety were tested in different clinical studies.
In a group of 38 patients with peripheral neuropathy of various etiologies treated for 4 weeks with R-LA liquid formulation a significant reduction in the total value obtained using NPS (Italian Neuropathic Pain Scale) was registered [29]. Despite performed on a limited number of patients, this study confirms that the liquid solution of R-LA provides relief from the pain symptoms of peripheral neuropathy. Beside confirming the efficacy of LA in the relief of symptoms in neuropathic pain, the results obtained may be attributed also to the improved bioavailability of the new formulation. The aim of the present investigation is to confirm the high bioavailability of the liquid formulation Liponax® sol evaluating its pharmacokinetics parameters in human, considering that according to literature, beneficial effects of LA were also correlated to a threshold of activation reached with a maximum concentration (Cmax) of 4-5 μg/mL and an area under the curve (AUC) of 171 min × μg/mL (Hermann et al., 1996). For this purpose, Liponax® sol was administered in fasting healthy adult at 300 mg dose of R-LA.
Materials and Methods
Study design
To evaluate pharmacokinetic parameters (Tmax, Cmax, AUC and T1/2) a clinical study was performed by Comegen Medical Cooperative (Naples, Italy) on a healthy adult population. Blood samples were collected after the consumption of an oral dose of a food supplement (Liponax® sol) containing 300 mg of R-LA after an overnight fast of 12 h. The subjects received oral and written information concerning the study before they gave their written consent. Protocol, letter of intent of volunteers, and synoptic document about the study were submitted to the Scientific Ethics Committee of ASL NA 1, Naples, Italy. The study was approved by the Ethics Committee and the chairperson was Silvana Perna (Protocol N°122). The study was carried out in accordance with the Helsinki declaration of 1964 (as revised in 2000).
Materials
R-LA, naproxen (NA), acetic acid (analytical grade 99% pure), methanol (analytical grade 99% pure) and human heparinized plasma were purchased from Sigma Aldrich (Missuri, United States). Water and acetonitrile were LC/MS pure grade >99.9% and were purchased from Merck (Merck Company, Germany). Blood samples were collected in 10-mL EDTA coated tubes (Becton Dickinson, Plymouth, United Kingdom). Liponax® sol was supplied by I.B.N. SAVIO (via del Mare 36, Pomezia, 00040, Italy).
Sample preparation
R-LA stock solution (1mg/mL) was prepared in methanol and stored at 4°C. The calibration curve was obtained in a concentration range of 0.05 – 5.00 μg/mL with six concentration levels and performing triplicate analysis for each level. Naproxen (NA) was selected as internal standard at the concentration of 5μg/mL. All the solutions were stored at 4°C until the beginning of the analysis and there was no change in stability after 30 days (data not shown).
UHPLC-MS conditions
UHPLC-MS analyses were performed on an Acquity I class, equipped with a QDa single quadrupole mass detector (Waters, Milford (MA), U.S) system. The separation was performed on a Kinetex® Biphenyl 100 Å column with geometry (L × I.D) 10 cm × 2.1 mm, 2.6 μm (Phenomenex®, Bologna, Italy) employing as mobile phases: A) 0.1% CH3COOH in H2O and B) ACN, with the following gradient: 0 min, 45% B, isocratic for 2.50 min, 2.51 min, 99 % B, isocratic for 1 min. Returning to 45 % in 1.50 min. The flow rate was set to 0.4 mL/min. Column oven was set to 40°C, 5 μL of extract were injected.
The ESI was operated in negative mode. Source temperature was 600°C, Probe voltage -3.5 kV. Nitrogen was used as nebulizer gas (10 L/min). MS analysis were conducted in selected ion recording (SIR) mode, employing 205.0 m/z for R-LA and 229.0 m/z for NA.
Study population
Study participants were recruited by the Cooperative of physician, Comegen (Naples, Italy). Subjects of both sexes, aged 18-65 years, were considered eligible for enrolment if they are in healthy status with a BMI < 25 kg/m2. Pregnant women, women suspected of being pregnant, women who hoped to become pregnant, breastfeeding, subjects with R-LA allergy, smokers, subjects exposed to a high risk of cardiovascular events, subjects suffering from endocrine disorder (e.g. hypothyroidism, Cushing’s syndrome, polycystic ovary syndrome or PCOS), subjects taking corticosteroids, subjects who have taken food supplements in the two weeks before recruiting, subjects suffering from liver disease, and not self-sufficient were excluded from the study.
Blood samples (2.5 mL) for the determination of R-LA plasma concentration were collected at the time of 0, 2, 5, 10, 15, 20, 25, 30, 40, 50, 60, 90, 120, and 180 min after oral administration as reported in Figure 1.
Pharmacokinetic parameters
The pharmacokinetics parameters calculated for each subject included: maximum plasma concentration (Cmax) of R-LA after oral administration of Liponax® sol (10mL), time to reach maximum concentration (Tmax), area under the concentration–time curve (AUC) equivalent to the total amount of R-LA that reaches the systemic circulation unmodified, and elimination half-life (t1/2).
Method validation
Sample preparation: To an aliquot of 200 μL of plasma in a 1.5mL Eppendorf plastic tube, 30μL of the internal standard (100μg/mL) were added to achieve the concentration of 5μg/ mL. The solution was vortexed for 15 seconds. Then, 370μL of icecold acetonitrile were used for protein precipitation, vortexed for 30 seconds and centrifuged at 14.000rpm for 15 min. Thus, the supernatant was filtered (0.45μm pore size) and injected in the UPLC system.
Linearity: The linear regression was used to generate the calibration curve that correlates peak area versus analyte concentrations with values of correlation coefficient R2 ≥ 0.999. The peak areas were converted to the corresponding concentrations (μg/mL) and the amount of R-LA was expressed as μg of analyte per mL of plasma.
Precision, accuracy and absolute recovery: Precision was evaluated using the measurements of the repeatability (intraday) and intermediate precision (inter-day). Repeatability was established by five replicate injections of sample and solutions at low, medium, and high concentration levels of the calibration curve with the same chromatographic conditions and analyst at the same day and within three consecutive days. The results are expressed as the relative standard deviation percentage of the measurements (R.S.D. %). Accuracy of the method in term of recovery was measured by comparing the peak area of the spiked samples at three different concentration levels. Accuracy is given as a percentage of the recovered amounts, comparing experimental peaks with those obtained from the calibration curve. Limits of detection (LODs) and quantification (LOQs) were calculated by the ratio between the standard deviation (SD) of the regression line and analytical curve slope multiplied by 3.33 and 10, respectively.
Specificity and matrix effect
The specificity was evaluated using blank plasma samples from healthy subjects to assess the interferences. The matrix effect was estimated through the comparison of the results obtained from analysis of standard R-LA solutions at different concentrations ranging from 0.5 μg/ml to 5 μg/ml and R-LA spiked post-extracted plasma samples at the same concentrations. The matrix effect was calculated by the following formula:
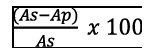
Where, as was the area under peak of the standard R-LA solution and Ap was the area under peak of the R-LA occurring in post-extracted plasma samples.
Results
The first aim of this study was to develop an ultra-high performance liquid chromatographic coupled with mass spectrometer (UHPLC-MS) method useful to determine the concentration of R-LA in human plasma after the oral consumption of Liponax® sol in healthy subjects. Figure 2 shows Single Ion Recording (SIR) chromatograms of R-LA and Naproxen obtained from the analysis of the standard compound in methanol (A), spiked plasma sample (B) and plasma from a healthy subject who consumed Liponax® sol (C). R-LA and NA peaks resulted to be well separated under the used experimental conditions. R-LA and NA were identified using the standard compound retention times (1.12 and 1.33 min, respectively) and m/z values obtained from parent ions (for R-LA m/z [M-H]- = 205.0, for NA m/z [M-H]- = 229.0).
Linearity
To determine R-LA plasma concentration, a calibration curve was obtained in a concentration range of 0.05 – 5 μg/mL with six concentration levels and performing triplicate analysis for each level. Naproxen was selected as internal standard in the concentration of 5 μg/mL. Linear regression analysis showed a high correlation coefficient (R2 of 0.9997; y = 0.1257x - 0.0033), proving a good linearity of the method in the selected concentration range.
Precision, accuracy and absolute recovery
The values obtained from intra- and inter-day precision and accuracy showed good repeatability in terms of retention time and concentration, as reported in Table 1. Intra-day RSD % value relative to R-LA spiked in human plasma, ranges from 1.174 to 1.880 and inter-day RSD % value ranges from 1.694 to 3.914, respectively (Table 2).
The R-LA absolutely recovery from plasma was 120.00 %, 105.21 % and 102.23 % at the concentrations of 0.5, 1.0 and 5.0 μg/mL, respectively. The matrix effect calculated, as reported in materials and method, resulted to be on average 21%. The limits of detection (LOD) and the limit of quantification (LOQ), calculated as reported in Material and Method, were 0.012 and 0.038 μg/mL, respectively.
Pharmacokinetic study
A total of 10 healthy subjects (6 female and 4 male) were included in the pharmacokinetic study. The subjects were recruited one a day for 10 days in fasted state. Each subject consumed 10 ml of Liponax® sol in an outpatient setting. Then blood samples were collected at the time of 0, 2, 5, 10, 15, 20, 25, 30, 40, 50, 60, 90 and 180 min after the oral administration of the food supplement. The blood samples were prepared as reported above. The mean plasma concentration versus time profile of R-LA following a single 300 mg oral dose in 10 subjects were shown in Figure 3. As reported in the Table 3, R-LA is rapidly absorbed as highlighted by the Tmax value of 11 min. Moreover, R-LA reaches high plasma concentrations with a mean Cmax of 8 μ/ mL. R-LA distribution in various tissues and its clearance confirm the plasma pharmacokinetic profile shown in Figure 3.
Discussion
LA is a strong antioxidant compound widely used in oxidative stress-related clinical conditions (e.g. diabetic complications, mechanical compression neuropathies, neurodegenerative and cardiovascular pathologies, physical and mental impairment, obesity, etc) [11,24,29-33]. Despite its beneficial properties, standard solid LA formulations for oral use have pharmacokinetic limitations such as high Tmax and low bioavailability (Table 4).
This study evaluates the pharmacokinetic profile of the patented oral liquid formulation containing 300 mg of the active enantiomer R-LA, (Liponax® sol), in humans. The obtained findings are in agreement with pharmacokinetic data obtained with a liquid formulation of R-LA in Sprague-Dawley rats [18] and are consistent with data obtained from the study on LA saline solution for injection [38] confirming both the efficacy and the improved PK parameters of the R-LA liquid formula. Carlson and co-workers achieved the similar PK results in humans using freshly dissolved R-LA Na salt in water that is not suitable as commercial formula due to taste and stability issue [39]. One explanation could be that at low concentrations LA is actively transported by intestinal protein carriers, such as the monocarboxylate transporter usually involved for medium-chain fatty acids absorption [28]. On the other hand, at high concentration the passive diffusion mechanism is favored for absorption [40]. R-LA contained in Liponax® sol thanks to the innovative and patented formulation is completely dissolved in solution, stable over the time and in the gastric environment [27]. While the dissolution of traditional tablets represents a crucial passage in absorption with an intense hepatic metabolism [35], the immediate availability of R-LA in Liponax® sol liquid formulation allows the transient saturation of the first-pass metabolism in the liver showing an high Cmax. Furthermore, the new formulation stabilizes and makes readily available the only active enantiomer R-LA, without any possible impact on PK value by the S-LA [18,19]. As reported in a recent pharmacokinetic study [41], LA concentrations accumulated in the cells were directly proportional to the plasma levels with an increase of cellular glutathione levels and a decrease of the oxidative stress. The approach proposed by these authors for eliciting antioxidant activity at the cellular level is the use of a formulation allowing the compound to reach its target at highest concentration and in the shortest time. According to literature data, LA results to be bioactive if reaches a threshold of activation corresponding to a Cmax of 4-5 μg/mL and AUC of 171 min × μg/ mL [42]. In light of this, the pharmacokinetics parameters of 300 mg R-LA dose contained in Liponax® sol promise better beneficial results and, the liquid formulation could be a great alternative to tablets with better patient.
A summary of the pharmacokinetic parameters of several studies with healthy human volunteers is reported in Table 4. The methods of analysis adopted in these studies are similar. Most investigations studied the pharmacokinetic of tablets containing 600 mg of racemic mixture (R-S), whereas the liquid formula tested here contained only 300 mg of the form R-LA. Liponax® sol showed an improved of Cmax and a very high AUC. In 2011 Mignini’s et al. [27] demonstrated that a tablet consisting of 600 mg racemic of LA and B complex vitamins, manufactured with a patented technology adopted also for other drugs, containing surfactants, super disintegrating, maltodextrins and lecithin, showed better results than traditional LA tablets but a Cmax lower than that found for Liponax® sol. This approach demonstrated that new technologies aimed to increase the absorption of LA are important to obtain better pharmacokinetic parameters and consequently to improve the clinical efficacy.
Taken together, these findings could explain the beneficial activity of the liquid formulation of R-LA previously showed in diabetic rats with neuropathy [18] and in different cases of neuropathies in humans [28], strengthening the use of Liponax® sol as a strong antioxidant compound for numerous diseases related to oxidative stress. These results are promising and encourage us to perform further studies to confirm the R-LA new liquid formulation beneficial effects.
In conclusion, the liquid formula for oral use containing 300 mg of R-LA tested in human healthy voluntaries, showed better pharmacokinetic parameters with a rapid absorption, high bioavailability, and optimized half-life. The comparison of literature data on pharmacokinetic parameters of traditional LA tablets show that, in our experimental conditions, this liquid oral formulation demonstrated to overcome the limitations ascribed to oral lipoic acid supplementations. These promising pharmacokinetic parameters will induce us to perform further clinical trials to show the beneficial effects for the consumption new liquid R-LA formulation.
To Know more about Open Access Journal of Toxicology
Click here: https://juniperpublishers.com/index.php




No comments:
Post a Comment