Dermatology & Cosmetics - Juniper Publishers
Abstract
Background: Skin health is becoming increasingly important and individuals see both ingested supplements and topical treatments as important ways to help skin attributes.
Aims: The main aim of this prospective, randomized, placebo-controlled study was to determine the effects of a unique supplement containing collagen peptides, lutein, and wheat lipid extract containing ceramides on skin appearance and attributes. Additional aims of the study were to determine if using a facial cleansing device along with the supplement provided complimentary benefits on skin apperance and to monitor safety paramters to confirm safety of the supplement.
Methods: Sixty healthy adults between the ages of 40 and 60 years with Fitzpatrick skin types I and II were recruited to participate in the study. The primary variables measured were changes in skin attributes including skin color and skin carotenoid levels and visible results as assessed by a dermatologist and self-assessment between groups given supplement (collagen peptides, ceramides, and lutein (CCL)) or placebo.
Results: Skin carotenoid levels significantly increased, with supplementation, from baseline to 30 and 120d. An increase was found in facial skin color (b*) at 30 and 120d with CCL while placebo treatment was unchanged between baseline and 30d, but then increased between 30 and 120d. Significant improvements in facial skin attributes were found by dermatologist assessments and self-assessments for CCL, cleansing device, or the combination.
Conclusions: Supplementation for 120d with CCL with and without a cleansing device supported skin health and improved several skin attributes.
Keywords: Collagen; Ceramides; Lutein; Skin Abbreviations: CCL: Collagen Peptides, Ceramides, and Lutein; RIUs: Raman Intensity Units
Introduction
Providing the necessary important nutrients through the foods and supplements we consume is important for maintaining healthy skin. Skin appearance gets worse with age and consumption of supplements have been found to improve skin health, but there is a limited number of studies to support these claims. The supplement delivered in the study provided collagen peptides, wheat lipid extract containing ceramides, and lutein (CCL). Each of these ingredients has been demonstrated to individually improve skin health but have not been studied together. Collagen peptides have been shown to clinically improve skin attributes such as skin texture, decrease area of skin ultraviolet damage, increase skin hydration, and improve visible skin results such as hydration, elasticity, wrinkles, and roughness [1,2]. Ceramides derived from wheat have been shown to improve skin texture, increase skin hydration, and improve visible skin attributes such as hydration, elasticity, and wrinkles [3]. Human skin has an inherent antioxidant capacity to reduce the potential damage caused from oxidative stress. The loss of this natural skin antioxidant capacity has been demonstrated to be counteracted by oral administration of carotenoids, such as lutein. Lutein is an antioxidant and previous studies with lutein supplementation have shown a positive impact on skin attributes such as improved skin radiance and hydration, and protection from ultraviolet light [4-6]. Topical skin treatments are also used to improve the appearance of skin. Individuals see both topical treatments and ingested supplements as important ways to improve skin health. In this study we had all the subjects use a facial cleansing device on half of their face for the duration of the study. The cleansing device gently cleanses and exfoliates the skin. The objective of this study was to investigate the impact a supplement blend of collagen peptides, lutein, and wheat lipid extract containing ceramides has on skin for 120d supplementation with and without a cleansing device. To our knowledge, a mixture of collagen peptides, lutein, and wheat lipid extract has not been studied for improvements on skin health. Furthermore, another objective was to determine if CCL along with the cleansing device had improved or complementary benefits to skin appearance compared to each treatment individually.
Materials and Methods
Subjects
Healthy, nonsmoking men and women between the ages of 40 and 60 years of age and body mass index (BMI) between 19 and 32 (kg/m2) with Fitzpatrick skin types I and II were recruited to participate. Subjects had to present with moderate signs of skin aging based on dermatologist assessment to be considered for enrollment. Exclusion criteria included: history of chronic diseases, skin diseases or abnormalities; regular consumption of dietary supplements for skin, nail, and hair health or containing carotenoids, collagen, or ceramides; or had used an anti-aging skin care treatment within 30d of study enrollment. Subjects who were pregnant, nursing, or planning to become pregnant were also excluded. Subjects were randomized into either the supplement or placebo groups in a 2:1 ratio (CCL n=40, placebo n=20). The study was approved by an Institutional Review Board (Allendale Institutional Review Board (AIRB), Old Lyme, CT) and conducted according to Helsinki Declaration as revised in 1983. All subjects signed an informed consent document prior to enrollment. The study was registered on ClinicalTrials.gov (NCT03771807).
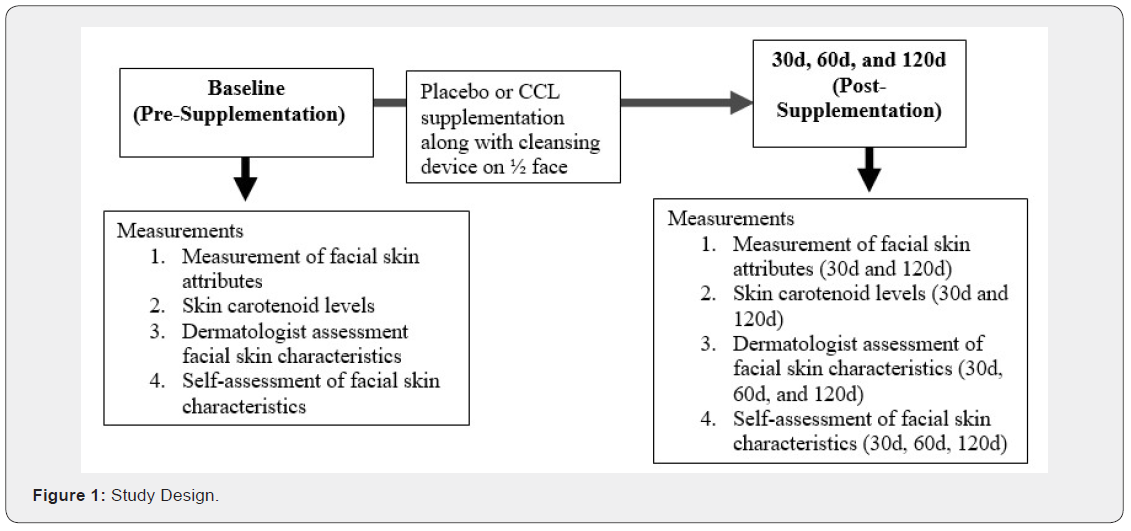
Study design
This study was a single center, prospective, randomized, double-blind, placebo-controlled study to investigate the effects of 120d supplementation with placebo or CCL supplement on skin carotenoid levels and facial skin attributes. The initial study design only planned for 90 days of supplementation, but this study occurred during the COVID-19 pandemic. The 90d clinic visit was not able to be completed due to lockdowns in the state of North Carolina (the location of the study). Therefore, the study was extended for an additional 30d, so that the subjects were able to complete the end of study visit at 120d and all skin attributes could be assessed. All the subjects were also given a facial cleansing device (LumiSpa, Nu Skin Enterprises, USA) to use daily on half of their face to determine if the facial cleansing device positively influenced the skin more than the supplement alone. A cleanser was used on the entire face along with the cleansing device on all subjects. The primary endpoints were skin attributes (color and skin carotenoid levels) and visible attributes as assessed by a dermatologist and self-assessment (lines, firmness, radiance, texture and overall appearance) between groups given the supplement or placebo. Subjects visited the clinical site at screening, baseline, 30d, 60d, and 120d. Due to COVID-19, subjects were not able to enter the clinical facility at the 60d time-point, but were able to have visible skin assessment by a dermatologist and complete self-assessment (lines, firmness, radiance, texture and overall appearance). At the 60d visit subjects were also asked about adverse events and unused study product was collected and compliance was calculated. Safety was also assessed by completing basic blood chemistry and complete blood count (CBC) analyses, and taking weight, heart rate, and blood pressure measurements at baseline and end of study. All measurements were able to be collected at baseline, 30d, and 120d visits.
Supplement
A placebo or supplement was delivered in sachets. Subjects were instructed to consume one sachet of placebo or CCL supplement mixed in 8-16 ounces water and consume orally once daily for 120d. Each daily supplement sachet delivered 2.5g collagen hydrolysate (VERISOL, GELITA, Germany), 70mg wheat lipid extract containing ceramides (Ceratiq, Epi Ingredients France), and 5mg lutein (FloraGLO, Kemin Industries, Inc.). Additional ingredients included silicon dioxide, stevia extract, citric acid anhydrous, natural orange flavor, bitter blocker, and beta-carotene. Placebo sachet contained maltodextrin (2.6g) along with the same levels of silicon dioxide, stevia extract, citric acid anhydrous, natural orange flavor, and beta-carotene that are in the active product. The placebo and active supplement were manufactured under good manufacturing practices by Nu Skin Enterprises (Provo, UT). Stability testing showed that the active ingredients of collagen peptides and lutein were maintained from manufacturing and for the duration of the study. The amount of wheat extract oil containing ceramides in the formulation was based on input at the time it was manufactured. Supplements were maintained at room temperature and distributed to each subject. Compliance was determined by the number of sachets distributed and the number returned by subjects. Adverse reactions as defined as any untoward medical occurrence (sign, symptom, or laboratory finding), regardless of severity and whether or not attributed to the supplements were reported to the physician/researcher.
Skin Carotenoid
Skin carotenoids levels were measured noninvasively in the palm of the hand using Raman spectroscopy (BioPhotonic Scanner, NSE Products, Provo, UT) as described in other clinical studies [7-10]. Each subject placed their hand firmly over the infrared optical port. Each subject was measured twice at each visit and measurements were recorded in Raman Intensity Units (RIUs). In the event the two measurements had a greater difference than 3,000 RIUs, a third measurement was taken, and the values were averaged.
Skin Color
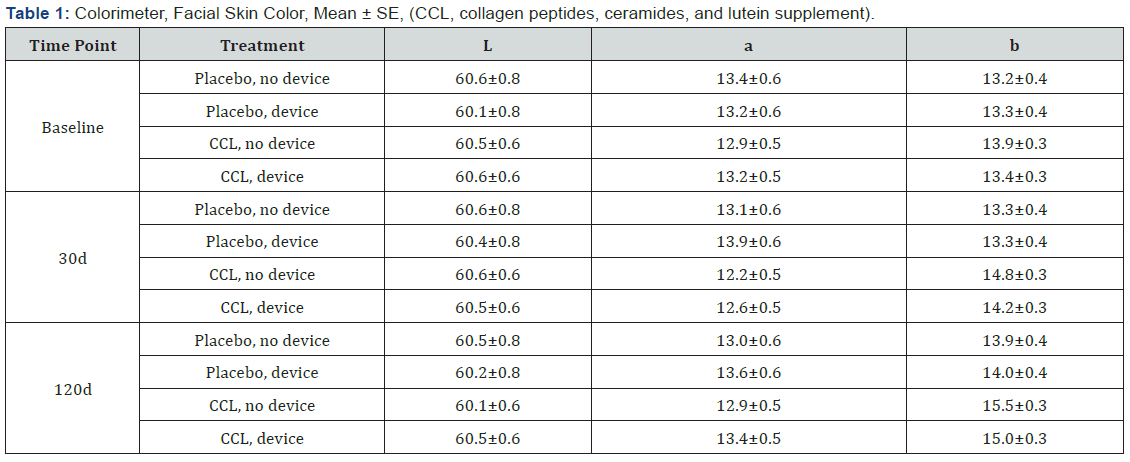
A colorimeter (Minolta) was used to evaluate facial skin color. This instrument allowed evaluating the skin color using the chromatic space L*a*b*. This data represents the luminance (L*), the quantity of red (a*:red-green axis), and the color intensity (b*: yellow-blue axis). Skin color was measured on each side of the face on the cheek.
Dermatologists and Subject Facial Assessments
A board certified dermatologist who was blinded to study participants randomization assessed facial skin characteristics. Skin characteristics included lines, firmness, radiance, texture/smoothness, and overall appearance using a 5 point ordinal scale (0=best and 4=worst) was assessed. Subjects also completed a self-assessment survey of facial skin characteristics (lines, firmness, radiance, texture/smoothness, and overall appearance) using a similar 5 point ordinal scale. Both dermatologist and subject skin grading occurred at baseline, 30d, 60d, and 120d. Assessments were completed on both sides of the face.
Statistical Methods
The analysis method applied to carotenoid and colorimeter data was Repeated Measures Analysis of Variance with Treatment and Time as the experimental factors. For the metrics that involved the face there is the additional cleansing device experimental factor in the analysis. The analysis performed on the dermatologist and subjects’ assessments on facial parameters (skin characteristics) was a Friedman Test and followed up with Steel-Dwass “All Pairs procedure” to identify significant effects for those time points that significantly differed. These analyses used JMP statistical software. P-values less than or equal to 0.05 were considered significant. An additional ordinal logistic regression was carried out on the various metrics for the expert and subject assessments. There was a “split plot” aspect to the analysis since the CCL vs. placebo is between subjects effect while the cleansing device effect and the time are within subject effects. The data is reported as predicted probabilities that a subject would have a rating that is 2 or lower over the probability that the rating is 3 or greater at baseline and after 120d. The analysis was conducted using R, a statistical programming language and computing environment, and the ordinal, rcompanion, and RVAideMemoire statistical libraries. The cumulative link mixed models (clmm) function from the ordinal library was used to fit models to the ordinal responses from the subject and expert assessments. A process of model fitting started with a model that includes all main effects and interactions for (CCL versus Placebo) Treatment, Device (Yes or No) and Time (measured at 0, 30, 60, and 120 days and modeled as a continuous predictor variable). The model fitting process began with all main effects and interactions included and then non-significant higher order effects were removed and the new models refit until no further simplifications was possible using the 0.05 criterion. P-values less than or equal to 0.05 were considered significant and less than 0.1 were considered a trend.
Results
Sixty healthy subjects (n=8 males and n=52 females) were enrolled and completed the study. In the placebo group there were 2 males and 18 females. In the CCL group there were 6 males and 34 females. The average age of subjects in the placebo group was 52.7 ± 7 years and the average age of subjects in the CCL group was 51.3 ± 6 years. Although the average age of the placebo group was slightly higher than the CCL group, the age of the subjects were similar between the placebo and CCL groups. This difference is not thought to impact the study outcomes. All subjects had ethnicity of Non-Hispanic/Latino. 59 subjects were white and 1 subject was black/African American. No subject withdrew from participation. The average CCL supplement compliance was 99% and placebo compliance was 86%. There were no adverse events reported during the conduct of the study. The study was run during the COVID-19 pandemic, but no subjects were diagnosed with COVID-19 during the study. All vital measurements and blood laboratory values were normal or not clinically significant if abnormal in both placebo and CCL supplement groups. The supplement demonstrated an excellent safety profile .
Skin Carotenoid Concentrations
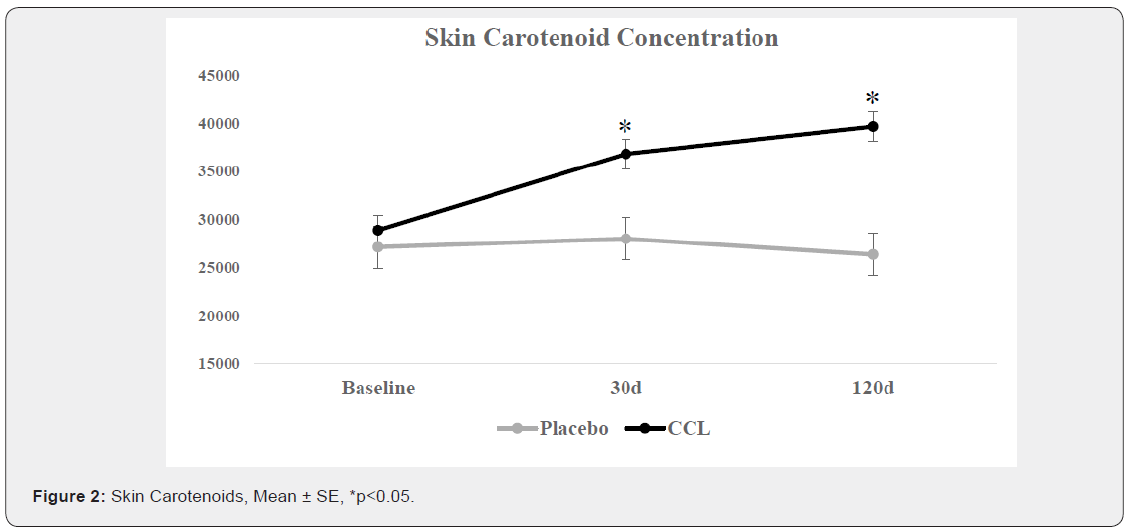
The treatment effect of CCL supplementation (p=0.0022), the time effect (p< 0.0001), and their interaction (p< 0.0001) were all significant. The average level for the CCL treatment was equivalent to the placebo at baseline but was significantly greater at 30 and 120d. The average skin carotenoid score for the placebo group at baseline was 27,950 RIUs. After 30d the average score in the placebo group was 28,000 RIUs and then slightly decreased to 26,350 RIUs after 120d. The average skin carotenoid score for the CCL group at baseline was 28,900 RIUs. After 120d, there was a significant increase to an average score of 39,650 RIUs. The mean increase at 120d from baseline was 10,750 RIUs (Figure 2).
Colorimeter
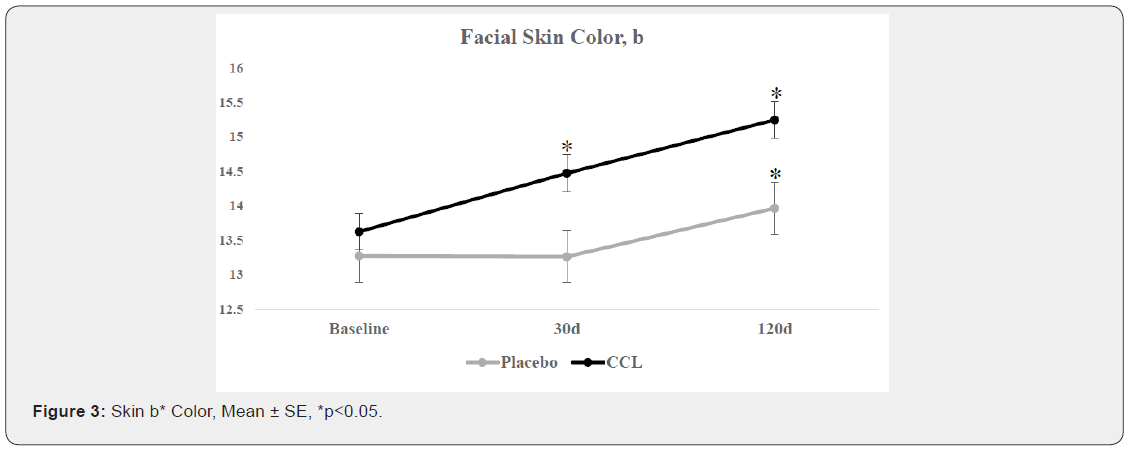
Colorimeter measurements were taken on both sides of the face (Table 1). For face L* value, none of the experimental effects were statistically significant. For face a* value, there were two significant effects for this variable, a main effect for cleansing device (p=0.0209) and a treatment by time interaction (p=0.0412). For face b* value (Figure 3), there were two significant interaction effects, cleansing device x CCL (p=0.0281) and treatment x time (p=0.0049). The CCL supplement treatment showed an increase in the mean level across the study while the placebo treatment was unchanged between baseline and 30d, but then increased between 30 and 120d. The device by treatment interaction indicates that the cleansing device treatment reduces the differences between the CCL and placebo treatments.
Dermatologists and Subject Assessments
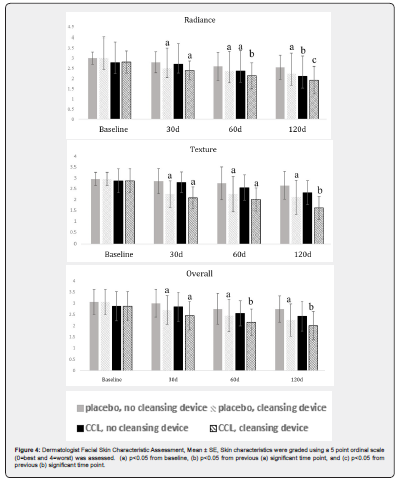
Significant results for the dermatologist facial skin grading are shown in Figure 4. For dermatologist grading of radiance, the Placebo-Cleansing Device treatment resulted in a significant Friedman Test, p = 0.0001. The Steel-Dwass test showed significant improvement at 30d which was maintained at 60 and 120d. The CCL-Cleansing Device group resulted in a significant Friedman test, p < 0.0001. There was a significant improvement at each time point after baseline. The CCL-No Cleansing Device treatment resulted in a significant Friedman Test, p < 0.0001. The Steel-Dwass test showed improvement at 60 and 120 days but not at 30 days. The Placebo-No Cleansing Device grading of radiance was not changed throughout the study. For dermatologist grading of texture, the Placebo-Cleansing Device group had a significant Friedman test p-value, p < 0.0001. The Steel-Dwass test showed significant improvement from baseline to 30d, which was maintained at 60 and 120d. The CCL-Cleansing Device group showed a significant Friedman test, p < 0.0001. The Steel-Dwass test showed significant improvement at 30d, which was maintained at 60d, and then another significant improvement at 120d. The Placebo-No Cleansing Device and CCL-No Cleansing Device groups showed no significant changes. For overall dermatologist facial grading, the Friedman Test p-value was 0.0476. The placebo, cleansing device group had a significant improvement at 30d which was maintained at 60 and 120d. The CCL-Cleansing Device group had a Friedman Test p-value less than 0.0001. The Steel-Dwass test showed that there was a significant improvement from baseline to 30d, another significant improvement from 30d to 60d which was maintained at 120d. The Placebo-No Cleansing Device and CCL-No Cleansing Device groups showed no significant changes. For the Friedman Test, expert assessments for lines and firmness were not significantly changed. The subject assessments for lines, firmness, radiance, texture, and overall were not significantly changed during the study using this statistical analysis.
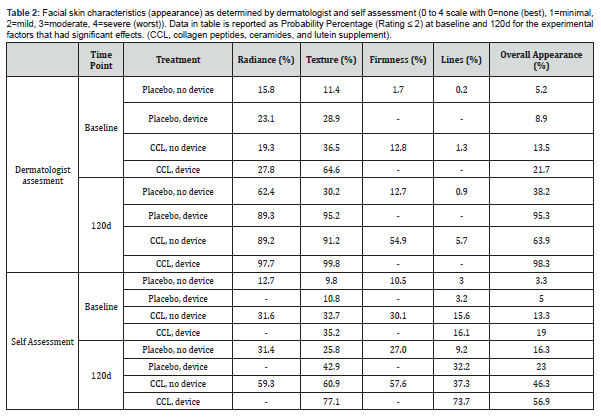
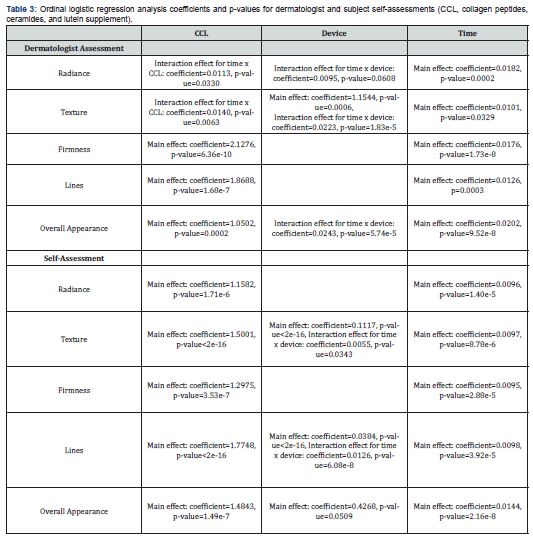
In order to make a more comprehensive evaluation of the dermatologist’s and subjects’ assessments an ordinal logistic regression analysis was also made of the data. This approach found that there were enhanced skin benefits when using both supplement and device compared to each treatment alone. The coefficients and p-values of this analysis are reported in Table 3, and the probability percentages are reported in Table 2. For the dermatologist’s assessments, the blend treatment increased the probability of a subject being rated a 2 or lower after 120 days for skin radiance, texture, lines, firmness, and overall health. For example, for dermatologist’s ratings, the use of the device for 120 days would result in 89% of people achieving a score of 2 or lower for skin radiance. The use of CCL for 120 days would result in 89% of people achieving a score of 2 or lower for skin radiance. Additionally, the use of the cleansing device and CCL together would result in 98% of people achieving a score of 2 or lower. For the dermatologist’s ratings, the use of the device for 120 days would result in 95% of people achieving a score of 2 or lower for skin texture. The use of CCL for 120 days would result in 91% of people achieving a score of 2 or lower for skin texture. The use of the device and supplement together would result in 99.8% of people achieving a score of 2 or lower for skin texture. The subjects’ self-assessments found that the CCL treatment increased the probability of a subject being rated a 2 or lower after 120 days for skin radiance, texture, lines, firmness, and overall health. The device increased the probability for lines, texture, and overall health. Specifically, for lines, subjects using CCL for 120 days would result in 37.3% of people achieving a score of 2 or lower for skin lines, compared to only 15.6% at baseline. Additionally the use of CCL and device together would result in subjects achieving a score of 2 or lower after 120 days for skin lines in 73.7% of people compared to 16.1% at baseline.
Discussion
In the present study it was found that 120d supplementation resulted in increased skin carotenoid levels (antioxidant protection) as well as observable improvements in skin characteristics by a dermatologist’s assessments. Skin health was visually improved which could be related to a change in the color of the skin. Carotenoids are pigments that are yellow/orange, which were shown to significantly increase in the skin during this study. The yellowness of the skin (b* value) also showed a tendency to increase on the face. This change in color as measured by a colorimeter could be attributed to skin carotenoid concentration. This change in skin color could also contribute to the improvement in radiance of the facial skin in the dermatologist’s assessment. A significant improvement in skin characteristics as determined by the dermatologist’s assessment of radiance, texture, and overall appearance following supplementation with and without cleansing device was found using the Friedman Test and Steel-Dwass All Pairs procedure. For radiance, the CCL supplement alone took until the 60d and 120d time-points to have significance compared to baseline. However, the cleansing device alone did show significant improvement in radiance at 30d which was maintained but not further improved at 60d and 120d. The CCL supplement along with the cleansing device resulted in significant improvement in radiance at 30d which was further improved at 60 and 120d. Combining the CCL supplement with the cleansing device led to improved radiance outcome. These improvements in the present study are attributed to the ingredients included in the CCL and the use of the cleansing device.
The ordinal logistic regression that was used to analyze dermatologist and self-assessments for skin health found that there were enhanced skin benefits when using both supplement and device compared to alone. For the expert assessments, CCL increased the probability of a subject being rated a 2 or lower after 120 days for skin radiance, texture, lines, firmness, and overall health. The device increased the odds for radiance, texture, and overall health. The probability of subjects having mild to no signs of negative skin attributes at baseline was 28% for radiance, 65% for texture, 13% for firmness, and 22% for overall appearance. The probability of subjects having mild to no signs of aging at the end of the study in the group using CCL and cleansing device was 98% for radiance, 99% for texture, 55% for firmness, and 98% for overall appearance. Additionally, the subject self-assessments found that the supplement treatment increased the probability of a subject being rated a 2 or lower after 120 days for skin radiance, texture, lines, firmness, and overall health. The device increased the probability for lines, texture, and overall. From this analysis it can be concluded that participants using CCL and cleansing device had a high probability of showing mild to no signs of aging after four months of using these products. This indicates the subjects had better skin appearance compared to baseline.
A limitation of this study is the limited amount of time subjects took the supplement. In the current study, facial skin elasticity and skin hydration were not significantly changed with the treatments. It is possible that a longer period of supplementation was needed to see significant benefits in skin elasticity and moisturization in this healthy population. Another limitation of this study was a skin biopsy was not taken. It is possible that changes were happening at the cellular level that were not quantifiable by the measurements taken in current study. For example, a double-blind, placebo-controlled study with 114 women aged 45-65 years were randomized to receive 2.5 g of collagen peptides (VERISOL, porcine) or placebo, daily, with 57 subjects allocated to each treatment. A subgroup had suction blister biopsies analyzed for procollagen I, elastin and fibrillin. After 8 weeks of intake, there was a significantly higher content of procollagen type I (65%) and elastin (18%) in the collagen peptide treated group compared to placebo treated group. A 6% increase in fibrillin was found after collagen peptide intake but this effect failed to reach significance 1. Another limitation of this study was that it was designed to look at the blend of the formulation, and was not able to look at the effects of individual ingredients in the formulation. A focus of an upcoming study could be to look at the blend of ingredients along with the individual ingredients to better understand how each ingredient is contributing to skin health benefits. Compliance was exceptional in the current study as the average compliance was 99% in taking the CCL supplement based on sachet count. Additionally, the increase in skin carotenoid score confirmed the consumption of CCL as the scores increased significantly.
Conclusion
This study provides evidence that in a relatively short period of supplementation, 16 weeks, the supplement provided antioxidant protection (skin carotenoids) and influenced several facial skin characteristics by dermatologist assessment. This study demonstrated that nutritional supplementation can positively support skin attributes and health. The subjects experienced improvements in skin attributes and appearance over the course of the study. Furthermore, the supplement along with the cleansing device resulted in visually improved skin compared to either treatment alone.
To Know more about Dermatology & Cosmetics
Click here: https://juniperpublishers.com/index.php




No comments:
Post a Comment