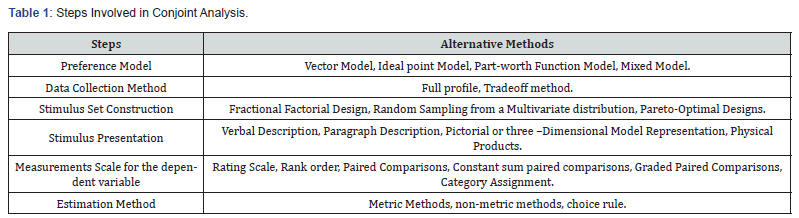
The study was aimed to evaluate the antioxidant and
anti-inflammatory biomarkers in heart tissues after treatment with the
Biofield Energy Treated Proprietary Test Formulation and Biofield Energy
Treatment per se to the animals on Cecal Slurry, LPS and E. coli-induced
systemic inflammatory response syndrome (SIRS) model in Sprague Dawley
rats. In this experiment, different antioxidants biomarkers such as
myeloperoxidase (MPO), superoxide dismutase (SOD), lipid peroxidase
(LPO) and proinflammatory cytokines such as tumor necrosis factor-α
(TNF-α), interleukin-6 (IL-6), macrophage inflammatory protein-2
(MIP-2), and matrix metallopeptidase 9 (MMP-9) were analysed using ELISA
assay in heart homogenate. A proprietary test formulation was
formulated including minerals (magnesium, zinc, calcium, selenium, and
iron), vitamins (ascorbic acid, pyridoxine HCl, vitamin E,
cyanocobalamin, and cholecalciferol), Panax ginseng extract, β-carotene,
and cannabidiol isolate. The constituents of the test formulation were
divided into two parts; one section was defined as the untreated test
formulation, while the other portion of the test formulation and the
animals received Biofield Energy Healing Treatment remotely for about 3
minutes by a renowned Biofield Energy Healer, Mr. Mahendra Kumar
Trivedi.
The level of MPO was reduced by 12.07% in the G6 (Cecal Slurry, LPS and E. coli
along with Biofield Energy Treatment per se to animals from day -15)
group as compared to the untreated test formulation (G4) group.
Moreover, the level of SOD was significantly (p≤0.05) increased by
19.03%, 17.26%, and 11.81% in the G6, G7, and G9 groups, respectively as
compared to the G4 group. The level of TNF-α was significantly
decreased by 25.97%, 40.28% (p≤0.01), 24.86%, 36.54% (p≤0.01), and
34.30% in the G5, G6, G7, G8, and G9 groups, respectively as compared to
the disease control (G2) group. Moreover, the level of IL-6 was
significantly (p≤0.001) decreased by 23.5%, 31.0%, 26.3%, and 39.8% in
the G5, G6, G8, and G9 groups, respectively as compared to the G2 group.
Additionally, the level of MIP-2 was reduced by 26.7% and 19.5% in the
G6 and G8 groups, respectively as compared to the G4 group.
Besides, the level of MMP-9 was significantly
(p≤0.001) reduced by 15.1%, 21.5%, and 34% in the G6, G8, and G9 groups,
respectively as compared to the G4 group. Altogether, the data imply
the antioxidant and anti-inflammatory potential of the Biofield Energy
Treated test formulation and Biofield Energy Treatment per se along with
preventive measure on the animal with respect to various inflammatory
conditions that might be beneficial various types of systemic
inflammatory disorders specially sepsis, trauma, septic shock or any
types of cardiac injuries. Therefore, the results showed the significant
slowdown the inflammation-related disease progression and its
complications/symptoms in the preventive Biofield Energy Treatment group
per se and/or Biofield Energy Treated Test formulation groups (viz. G6,
G7, G8, and G9) comparatively with the disease control group.
Keywords: Biofield Treatment; Inflammatory cytokines; The Trivedi Effect®; ELISA; SIRS; Antioxidant; Heart biomarker
Abbreviations:
SIRS: Systemic inflammatory response syndrome; MPO: Myeloperoxidase;
SOD: superoxide dismutase; LPO: Lipid peroxidase; CAD: Coronary artery
disease; LDL: Low-density lipoprotein; CAM: Complementary and
Alternative Medicine; NCCAM: National Center for
Complementary/Alternative Medicine; NCCIH: National Centre of
Complementary and Integrative Health; SD: Sprague Dawley; LPS:
Lipopolysaccharide; SEM: Standard error of mean; RA: Rheumatoid
arthritis; AD: Addison disease
Cardiovascular diseases are very common cause of
health burden worldwide [1]. Heart disease is the leading cause of death
for all age’s population in the United States. In 2010, coronary artery
disease (CAD) accounted for one in six deaths in the United States [2].
However, in 2020, one person dies every 36 seconds that’s one in every
four deaths in the United States from cardiovascular disease [3,4].
Oxygen free radicals promote low-density lipoprotein (LDL) peroxidation,
and increase the number of foam cells, that causes vascular endothelial
cell injury, and induce expression of proinflammatory cytokines [5].
Cytokines (TNF-α, TGF-β) and interleukins (IL-1, IL-4, IL-6, IL-8, and
IL-18) are responsible for the development of various inflammatory
pathologies of various vital systems such as cardiac, brain, renal,
lymphatic, etc. [6]. MIP-2 is produced by a variety of cells in response
to infection or injury. It is regulated by multiple factors like by
signalling through Toll-like receptor 2 (TLR2), TLR3, and TLR4 in
response to diverse pathogens [7]. Superoxide dismutases (SODs) an
antioxidant enzyme and also acts as a good therapeutic agent against
reactive oxygen species-mediated diseases [8]. Thus, in order to study
the change in heart cytokines in presence of Cecal Slurry, LPS and E. coli-induced
systemic inflammatory response syndrome model in Sprague Dawley rats, a
novel test formulation was designed with the combination of vital
minerals (selenium, zinc, iron, calcium, and magnesium), essential
vitamins (cyanocobalamin, ascorbic acid, pyridoxine HCl, vitamin E, and
cholecalciferol), and nutraceuticals (β-carotene, Ginseng, cannabidiol
isolate (CBD)). All the minerals and vitamins used in the test
formulation have significant functional role to provide vital
physiological responses [9,10]. Besides, cannabidiol itself has wide
range of pharmacological profile and has been reported to role in
different disorders [11,12], while ginseng extract is regarded as the
one of the best immune booster for overall immunity [13]. The present
study was aimed to evaluate the antioxidant and anti-inflammatory
potential of the Biofield Energy Treated Proprietary Test Formulation
and Biofield Energy Treatment per se to the animals on Cecal Slurry, LPS
and E. coli-induced systemic inflammatory response syndrome model in Sprague Dawley rats.
Biofield Energy Healing Treatment has been reported
with significant effects against various disorders and defined as one of
the best Complementary and Alternative Medicine (CAM) treatment
approach [14-16]. National Center for Complementary/Alternative Medicine
(NCCAM) recommended CAM with several clinical benefits as compared with
the conventional treatment approach [17]. National Centre of
Complementary and Integrative Health (NCCIH) accepted Biofield Energy
Healing as a CAM health care approach in addition to other therapies
such as deep breathing, natural products, Tai Chi, yoga, therapeutic
touch, Johrei, Reiki, pranic healing, chiropractic/osteopathic
manipulation, guided imagery, meditation, massage, homeopathy,
hypnotherapy, special diets, relaxation techniques, movement therapy,
mindfulness, Ayurvedic medicine, traditional Chinese herbs and medicines
in biological systems [18,19]. The Trivedi Effect®-Consciousness Energy
Healing Treatment was scientifically reported on various disciplines
such as in the materials science [20,21], agriculture science [22],
antiaging [23], gut health [24], nutraceuticals [25], pharmaceuticals
[26], overall human health and wellness. In this study, the authors want
to evaluate the effect of the Biofield Energy Treatment (the Trivedi
Effect®) on the given novel test formulation and Biofield Energy
Treatment per se to the animals on heart biomarkers in presence of Cecal
Slurry, LPS and E. coli-induced systemic inflammatory response syndrome model in in Sprague Dawley rats using standard ELISA assay.
Pyridoxine hydrochloride (vitamin B6), zinc chloride,
magnesium (II) gluconate, and β-carotene (retinol, provit A) were
purchased from TCI, Japan. Cyanocobalamin (vitamin B12), calcium
chloride, vitamin E (Alpha-Tocopherol), cholecalciferol (vitamin D3),
iron (II) sulfate, and carboxymethyl cellulose sodium were procured from
Sigma-Aldrich, USA. Ascorbic acid (vitamin C) and sodium selenate were
obtained from Alfa Aesar, India. Panax ginseng extract and cannabidiol
isolate were obtained from Panacea Phytoextracts, India and Standard
Hemp Company, USA, respectively. Dexamethasone was obtained from Clear
synth, India. For the estimation of heart antioxidant and inflammatory
biomarker panels, such as myeloperoxidase (MPO), superoxide dismutase
(SOD), lipid peroxidation (LPO), tumour necrosis factor alpha (TNF-α),
interleukin-6 (IL-6), macrophage inflammatory protein-2 (MIP-2), and
matrix metallopeptidase 9 (MMP-9) were procured from CUSABIO, USA using
specific ELISA kits.
Randomly breed male Sprague Dawley (SD) rats with
body weight ranges from 200 to 300 gm were used in this study. The
animals were purchased from M/s. Vivo Bio Tech, Hyderabad, India.
Animals were randomly divided into nine groups based on their body
weights consist of 10-12 animals of each group. They were kept
individually in sterilized polypropylene cages with stainless steel top
grill having provision for holding pellet feed and drinking water bottle
fitted with stainless steel sipper tube. The animals were maintained as
per standard protocol throughout the experiment.
Each ingredient of the novel test formulation was
divided into two parts. One part of the test compound did not receive
any sort of treatment and were defined as the untreated or control
sample. The second part of the test formulation was treated with the
Trivedi Effect® - Energy of Consciousness Healing Treatment (Biofield
Energy Treatment) by a renowned Biofield Energy Healer, Mr. Mahendra
Kumar Trivedi under laboratory conditions for ~3 minutes. Besides, three
group of animals also received Biofield Energy Healing Treatment (known
as the Trivedi Effect®) by Mr. Mahendra Kumar Trivedi under similar
laboratory conditions for ~3 minutes. The Blessing (prayer)/Treatment
was given to the test items/animals (present in the laboratory of Dabur
Research Foundation, near New Delhi, India), remotely from USA for about
3 minutes via online web-conferencing platform. After that, the
Biofield Energy Treated samples was kept in the similar sealed condition
and used as per the study plan. In the same manner, the control test
formulation group was subjected to “sham” healer for ~3 minutes
treatment, under the same laboratory conditions. The “sham” healer did
not have any knowledge about the Biofield Energy Treatment. The Biofield
Energy Treated animals were also taken back to experimental room for
further proceedings.
Seven days after acclimatization, animals were
randomized and grouped based on the body weight. The test formulation
was prepared freshly prior to dosing and administered to the animals
using an oral intubation needle attached to an appropriately graduated
disposable syringe. The dose volume was 10 mL/kg in morning and evening
based on body weight. The experimental groups were divided as G1 as
normal control (vehicle, 0.5% w/v CMC-Na); G2 as disease control (Cecal
Slurry, LPS and E. coli + 0.5% CMC-Na); G3 as reference item (Cecal Slurry, LPS and E. coli + Dexamethasone); G4 includes Cecal Slurry, LPS and E. coli along with untreated test formulation; G5 as Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation; G6 group includes Cecal Slurry, LPS and E. coli along with Biofield Energy Treatment per se to animals from day -15; G7 as Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation from day -15; G8 group includes Cecal Slurry, LPS and E. coli
along with Biofield Energy Treatment per se plus the Biofield Energy
Treated test formulation from day -15, and G9 group denoted Cecal
Slurry, LPS and E. coli along with Biofield Energy Treatment
per se animals plus the untreated test formulation. Dosing for groups G7
and G8 were started on Day -15 and continued till end of the
experiment. However, Group G1 to G5 and G9 animals were dosed with
respective formulations from Day 1 and continued till the end of the
experiment. Group G6 animals received Biofield Energy Treatment on
Day-15 and were not dosed throughout the experimental period. At the end
of the experimental period (8 weeks treatment), the animals were
sacrifice and heart were collected, homogenised, and the supernatant
subjected for estimation of antioxidants (MPO, SOD, and LPO) and
cytokines (TNF alpha, IL-6, MIP-2, and MMP-9).
A combination model of sepsis was developed in SD
rats by administering Cecal slurry (from donor animals,
intraperitoneally, at the dose of 400 mg/kg) in combination with LPS (at
the dose of 100 µg/animal) and E. coli [Escherichia coli; 0.2
mL (2M CFU)/animal]). The animals were monitored for various parameters
for up to 56 days after disease (SIRS) induction. Ten Donor (~20 weeks
old) rats were anesthetized. A midline laparotomy was performed on them
and the cecum was extruded. A 0.5 cm incision was made on the
anti-mesenteric surface of the cecum, and the cecum was squeezed to
expel the feces. The feces from different donor animals was collected
and weighed. Immediately after collection, the feces were pooled,
diluted 1:3 with 5% dextrose solution and filtered to get a homogeneous
suspension. Bacterial viability in the cecal slurry was analyzed. Cecal
slurry prepared from donor rats was injected intraperitoneally into
experimental rats (G2 to G9) at the dose of 400 mg/kg within 2 hours of
preparation. After 3 hours, lipopolysaccharide (LPS) at the dose of 100
µg/animal, and gram-negative viable bacteria such as E. coli [0.2 mL (2M CFU)/animal] were injected, intraperitoneally (G2 to G9).
With the continued treatment to the respective groups
of 8th week of the experimental period, all the animals were
sacrificed, heart were collected, homogenized and subjected for the
estimation of antioxidants and cytokines. The tissue from all the groups
was stored at -20°C for further estimation. Alternatively, aliquot all
the samples and store samples at -20°C or -80°C. Avoid repeated
freeze-thaw cycles, which may alter the level of cytokines during final
calculations.
The heart from all the groups was subjected for the
estimation of level of antioxidants such as MPO (CSB-E08722r), SOD
(706002), and LPO (700870) and cytokines such as TNF-α (CSB-E11987r),
IL-6 (CSB-E04640r), MIP-2 (CSB-E07419r), and MMP-9 (CSB-E08008r). All
the biomarker panel was estimation using ELISA method as per
manufacturer’s recommended standard procedure. This was a quantitative
method and the principle was based on the binding of antigen and
antibody in sandwich manner assay.
The data were represented as mean ± standard error of
mean (SEM) and subjected to statistical analysis using Sigma-Plot
statistical software (Version 11.0). For multiple comparison One-way
analysis of variance (ANOVA) followed by post-hoc analysis by Dunnett’s
test and for between two groups comparison Student’s t-test was
performed. The p≤0.05 was considered as statistically significant.
Estimation of Myeloperoxidase (MPO): Myeloperoxidase
(MPO) was estimated in the presence of the test formulation and the
data are graphically shown in Figure 1. The data suggested that the
disease control (Cecal Slurry, LPS and E. coli + 0.5% CMC-Na) +
0.5% CMC) group (G2) showed value of MPO as 2.41 ± 0.0.28 ng/mL, which
was increased by 0.45% as compared with the normal control (G1, 2.40 ±
0.1 ng/mL). However, positive control (Dexamethasone) treatment (G3)
showed the level of MPO in heart i.e., 2.80 ± 0.14 ng/mL.
The level of MPO in heart tissues was decreased by 9.98% and 12.07% in the G5 (Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation) and G6 (Cecal Slurry, LPS and E. coli
along with Biofield Energy Treatment per se to animals from day -15)
groups, respectively as compared to the untreated test formulation (G4)
group. High expression of MPO level in circulation are associated with
inflammation and increased oxidative stress that leads to cardiovascular
disease (CVDs) like coronary artery disease, congestive heart failure,
arterial hypertension, pulmonary arterial hypertension, myocardial
ischemia, stroke, cardiac arrhythmia and venous thrombosis [27].
Multiple lines of evidence suggested that MPO may play a role in
atherogenesis in humans. However, MPO has little role as
atheroprotective in the murine atherosclerosis model [28]. MPO plays an
important role in the host defense against different types of bacteria
and viruses. MPO is also an important enzyme in the inflammatory
process, and inflammation is a key component in the development and
progression of atherosclerotic and other forms of cardiovascular disease
[29]. Overall, in this experiment the Biofield Energy Treated test
formulation and Biofield Energy Healing Treatment per se reduced the
level of MPO in the heart tissues, which could be helpful for the
management of oxidative stress and inflammatory conditions related to
cardiovascular disorders.
Figure 1 The effect of the test formulation on the
level of heart myeloperoxidase (MPO) in Sprague Dawley rats. G1 as
normal control (vehicle, 0.5% w/v CMC-Na); G2 as disease control (Cecal
Slurry, LPS and E. coli + 0.5% CMC-Na); G3 as reference item (Cecal Slurry, LPS and E. coli + Dexamethasone); G4 includes Cecal Slurry, LPS and E. coli along with untreated test formulation; G5 as Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation; G6 group includes Cecal Slurry, LPS and E. coli along with Biofield Energy Treatment per se to animals from day -15; G7 as Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation from day -15; G8 group includes Cecal Slurry, LPS and E. coli
along with Biofield Energy Treatment per se plus the Biofield Energy
Treated test formulation from day -15, and G9 group denoted Cecal
Slurry, LPS and E. coli along with Biofield Energy Treatment
per se animals plus the untreated test formulation. Values are presented
as mean ± SEM (n=6-9).
Estimation of Superoxide Dismutase (SOD):
The effect of the test formulation and Biofield Energy Treatment per se
was assessed by estimating the level of heart superoxide dismutase
(SOD), and the results are graphically presented in the Figure 2. The
disease control (Cecal Slurry, LPS and E. coli + 0.5% CMC-Na) +
0.5% CMC) group (G2) showed value of SOD as 3.79 ± 0.13 U/mL, which was
decreased by 3.2% as compared to the normal control group i.e., 4.10 ±
0.18 U/mL. However, positive control (Dexamethasone) treatment (G3)
showed the level of SOD in heart i.e., 4.49 ± 0.22 U/mL, which was
increased by 13.3% as compared to G2.
The level of SOD was increased significantly by
1.27%, 19.03% (p≤0.05), 17.26% (p≤0.05), 6.15%, and 11.81% (p≤0.05) in
the G5 (Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation); G6 (Cecal Slurry, LPS and E. coli along with Biofield Energy Treatment per se to animals from day -15), G7 as Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation from day -15; G8 (Cecal Slurry, LPS and E. coli
along with Biofield Energy Treatment per se plus the Biofield Energy
Treated test formulation from day -15), and G9 (Cecal Slurry, LPS and E. coli
along with Biofield Energy Treatment per se animals plus the untreated
test formulation) groups, respectively with reference to disease control
group (G2). Further, the level of SOD was significantly increased by
3.8%, 22.01% (p≤0.05), 20.19% (p≤0.05), 8.80%, and 14.6% in the G5, G6,
G7, G8, and G9 groups, respectively with reference to untreated test
formulation group (G4). Studies in the heart suggest that extra-cellular
SOD is important for preventing oxidative injury after myocardial
infarction and may contribute to cardiac remodeling [30]. SOD is one of
the main intracellular antioxidant defence mechanisms is associated with
cardiac and vascular defects leads to hypertension and atherosclerosis.
It is also protecting thermogenesis [31]. Therefore, in this experiment
the Biofield Energy Treated test formulation significantly increased
the level of heart SOD, which could be beneficial inflammation and
oxidative damage.
Figure 2: The level of superoxide dismutase (SOD)
measured in heart tissue in Sprague Dawley rats after administration
with Biofield Treated test formulation and Biofield Treatment per se. G1
as normal control (vehicle, 0.5% w/v CMC-Na); G2 as disease control
(Cecal Slurry, LPS and E. coli + 0.5% CMC-Na); G3 as reference item (Cecal Slurry, LPS and E. coli + Dexamethasone); G4 includes Cecal Slurry, LPS and E. coli along with untreated test formulation; G5 as Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation; G6 group includes Cecal Slurry, LPS and E. coli along with Biofield Energy Treatment per se to animals from day -15; G7 as Cecal Slurry, LPS and E. coli + Biofield Energy Treated test formulation from day -15; G8 group includes Cecal Slurry, LPS and E. coli
+ Biofield Energy Treatment per se + Biofield Energy Treated test
formulation from day -15, and G9 group denoted Cecal Slurry, LPS and E. coli
along with Biofield Energy Treatment per se animals plus the untreated
test formulation. Values are presented as mean ± SEM (n=6-9). *p≤0.05
vs. G2 and #p≤0.05 vs. G4.
Estimation of Lipid Peroxidation (LPO): The
level of lipid peroxidation (LPO) end product in terms of
malondialdehyde (MDA) was detected in all the experimental groups and
the data are presented in Figure 3. The disease control (Cecal Slurry,
LPS and E. coli + 0.5% CMC-Na) group (G2) and positive control
(Dexamethasone) treatment (G3) groups showed value of MDA as 4.20 ± 0.48
µM and 4.33 ± 0.37 µM, respectively.
The level of MDA was decreased by 5.6%, 2.9%, and 18% in the G7 as Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation from day -15; G8 (Cecal Slurry, LPS and E. coli
along with Biofield Energy Treatment per se plus the Biofield Energy
Treated test formulation from day -15), and G9 (Cecal Slurry, LPS and E. coli
along with Biofield Energy Treatment per se animals plus the untreated
test formulation) groups, respectively with reference to disease control
group (G2). Additionally, the level of MDA was significantly reduced by
4.1%, 11.1%, 8.5%, and 22.8% (p≤0.05) in the G5 (Cecal Slurry, LPS and E. coli
along with the Biofield Energy Treated test formulation), G7, G8, and
G9 groups, respectively as compared to the untreated test formulation
group (G4). Oxidative stress and inflammation are two major mechanisms
leading to atherosclerosis. Under oxidative stress, phospholipids and
cholesterol esters can readily oxidized through a free radical-induced
lipid peroxidation (LPO) process to form a complex mixture of oxidation
products. These oxidized lipids are responsible for inflammatory
responses in atherosclerosis by interacting with immune cells
(macrophages) and endothelial cells [32]. The LPO products are highly
reactive and causes selective alterations in cell signaling, protein and
DNA damage, and cytotoxicity [33]. In this experiment, the Biofield
Energy Treated preventive groups significantly reduced the level of LPO
in heart tissues, which could be beneficial inflammation and oxidative
damage in heart.
Figure 3: The level of heart lipid peroxidation (LPO)
in Sprague Dawley rats after dosed with the Biofield Treated test
formulation and Biofield Energy Healing per se. G1 as normal control
(vehicle, 0.5% w/v CMC-Na); G2 as disease control (Cecal Slurry, LPS and
E. coli + 0.5% CMC-Na); G3 as reference item (Cecal Slurry, LPS and E. coli + Dexamethasone); G4 includes Cecal Slurry, LPS and E. coli along with untreated test formulation; G5 as Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation; G6 group includes Cecal Slurry, LPS and E. coli along with Biofield Energy Treatment per se to animals from day -15; G7 as Cecal Slurry, LPS and E. coli + Biofield Energy Treated test formulation from day -15; G8 group includes Cecal Slurry, LPS and E. coli
+ Biofield Energy Treatment per se plus the Biofield Energy Treated
test formulation from day -15, and G9 group denoted Cecal Slurry, LPS
and E. coli along with Biofield Energy Treatment per se animals
plus the untreated test formulation. Values are presented as mean ± SEM
(n=6-9). *p≤0.05 vs. G4.
Estimation of Tumour Necrosis Factor Alpha (TNF-α): The
expression of heart tumour necrosis factor alpha (TNF-α) in Sprague
Dawley rats after administration of Biofield Treated test formulation
and exposure of Biofield Treatment to the animals per se, and the
results are shown in Figure 4. The disease control (Cecal Slurry, LPS
and E. coli + 0.5% CMC-Na) group (G2) showed value of TNF-α as
204.12 ± 46.49 pg/mL, which was significantly (p≤0.01) increased by 399%
as compared with the normal control (G1, 40.91 ± 3.85 pg/mL).
Further, the positive control (Dexamethasone)
treatment (G3) showed significant (p≤0.01) decreased TNF-α level by 66%
i.e., 69.31 ± 8.52 pg/mL as compared to the G2 group. TNF-α level was
decreased significantly by 25.97%, 40.28% (p≤0.01), 24.86%, 36.54%
(p≤0.01), and 34.30% in the G5 (Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation); G6 (Cecal Slurry, LPS and E. coli along with Biofield Energy Treatment per se to animals from day -15), G7 as Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation from day -15; G8 (Cecal Slurry, LPS and E. coli + Biofield Energy Treatment per se + Biofield Energy Treated test formulation from day -15), and G9 (Cecal Slurry, LPS and E. coli
+ Biofield Energy Treatment per se animals + untreated test
formulation) groups, respectively as compared to the disease control
group (G2). Further, the expression of TNF-α was reduced by 16.2%,
10.9%, and 7.8% in the G6, G8, and G9 groups, respectively as compared
to the untreated test formulation group (G4). Pro-inflammatory cytokines
are consistently increased in congestive heart failure. In the
cardiovascular system, TNF-α activate signal transduction pathways may
causes vascular dysfunction, development, and progression of
atherosclerosis, and thus ultimately leads to myocardial infarction and
heart failure [34]. Another, study reported that TNFα is responsible for
the progression of heart failure as a mediator of myocardial
dysfunction and adverse remodeling, that leads to elevated levels of
circulating TNFα in heart failure patients as compared with the control
[35]. Moreover, TNF modulates both cardiac contractility and peripheral
resistance, the two most important haemodynamic determinants of cardiac
function [36]. Therefore, here the Biofield Energy Treated test
formulation and Biofield Energy Treatment per se significantly reduced
the level of TNF-α, which could be beneficial in the cardiovascular
disorders.
Figure 4: The expression of heart tumour necrosis
factor alpha (TNF-α) in Sprague Dawley rats after administration of
Biofield Treated test formulation and exposure of Biofield Treatment to
the animals per se. G1 as normal control (vehicle, 0.5% w/v CMC-Na); G2
as disease control (Cecal Slurry, LPS and E. coli + 0.5% CMC-Na); G3 as reference item (Cecal Slurry, LPS and E. coli + Dexamethasone); G4 includes Cecal Slurry, LPS and E. coli along with untreated test formulation; G5 as Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation; G6 group includes Cecal Slurry, LPS and E. coli along with Biofield Energy Treatment per se to animals from day -15; G7 as Cecal Slurry, LPS and E. coli + Biofield Energy Treated test formulation from day -15; G8 group includes Cecal Slurry, LPS and E. coli
+ Biofield Energy Treatment per se plus the Biofield Energy Treated
test formulation from day -15, and G9 group denoted Cecal Slurry, LPS
and E. coli along with Biofield Energy Treatment per se animals
plus the untreated test formulation. Values are presented as mean ± SEM
(n=6-9). ##p≤0.01 vs. G1 and **p≤0.01 vs. G2.
Estimation of Interleukin-6 (IL-6)
The expression of heart interleukin-6 (IL-6) in
Sprague Dawley rats after administration of Biofield Treated test
formulation and exposure of Biofield Treatment to the animals per se,
and the results are graphically shown in Figure 5. The disease control
(Cecal Slurry, LPS and E. coli + 0.5% CMC-Na) group (G2) showed
value of IL-6 as 19.05 ± 2.29 pg/mL, which was significantly (p≤0.001)
increased by 98.7% as compared with the normal control (G1, 9.59 ± 0.44
pg/mL). Further, the positive control (Dexamethasone) treatment (G3)
showed the level of IL-6 i.e., 10.46 ± 0.71 pg/mL, which was decreased
by 45.1% as compared to the G2 group. The level of IL-6 was
significantly decreased by 23.5% (p≤0.001), 31.0% (p≤0.001), 19.5%,
26.3% (p≤0.001), and 39.8% (p≤0.001) in the G5 (Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation); G6 (Cecal Slurry, LPS and E. coli along with Biofield Energy Treatment per se to animals from day -15), G7 as Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation from day -15; G8 (Cecal Slurry, LPS and E. coli
along with Biofield Energy Treatment per se plus the Biofield Energy
Treated test formulation from day -15), and G9 (Cecal Slurry, LPS and E. coli
along with Biofield Energy Treatment per se animals plus the untreated
test formulation) groups, respectively, as compared to the disease
control group (G2).
Further, the expression of IL-6 was decreased by
3.2%, 12.7%, 6.7%, and 23.9% in G5, G6, G8, and G9 groups,
correspondingly with reference to untreated test formulation (G4) group.
Based on the one of the studies from myocardial infarction which shows
that IL-6 signaling plays a causal role in cardiovascular disease [37].
The patients with high titre of circulating inflammatory biomarkers get
more susceptible to cardiovascular events. It is more common in patients
with high IL-6, associated with an increased incidence of myocardial
infarction and mortality among patients with acute coronary syndromes
[38]. There is an extensive body of the literature that supports that an
increased level of inflammatory cytokine like IL-6 is associated with
acute ischemic conditions and predictor of coronary artery disease [39].
Overall, in this experiment the Biofield Energy Treated test
formulation and Biofield Energy Treatment per se significantly reduced
the level of IL-6, which could be reduce the risks of inflammatory
diseases specially in the heart.
Figure 5: The expression of heart interleukin-6
(IL-6) in Sprague Dawley rats after administration of Biofield Treated
test formulation and exposure of Biofield Treatment to the animals per
se. G1 as normal control (vehicle, 0.5% w/v CMC-Na); G2 as disease
control (Cecal Slurry, LPS and E. coli + 0.5% CMC-Na); G3 as reference item (Cecal Slurry, LPS and E. coli + Dexamethasone); G4 includes Cecal Slurry, LPS and E. coli along with untreated test formulation; G5 as Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation; G6 group includes Cecal Slurry, LPS and E. coli along with Biofield Energy Treatment per se to animals from day -15; G7 as Cecal Slurry, LPS and E. coli + Biofield Energy Treated test formulation from day -15; G8 group includes Cecal Slurry, LPS and E. coli
+ Biofield Energy Treatment per se plus the Biofield Energy Treated
test formulation from day -15, and G9 group denoted Cecal Slurry, LPS
and E. coli along with Biofield Energy Treatment per se animals
plus the untreated test formulation. Values are presented as mean ± SEM
(n=6-9). ###p≤0.001 vs. G1 and ***p≤0.001 vs. G2.
Estimation of Macrophage Inflammatory Protein-2 (MIP-2): The
expression of macrophage inflammatory protein-2 (MIP-2) in heart tissue
after administration of the Biofield Treated/Blessed proprietary test
formulation and Biofield Energy Healing Treatment per se to the animals
was estimated, and the results are graphically shown in Figure 6. The
disease control (Cecal Slurry, LPS and E. coli + 0.5% CMC-Na)
group (G2) showed value of MIP-2 as 1734.78 ± 237.57 pg/mL, which was
decreased by 51.8% as compared with the normal control (G1, 3598.50 ±
395.77 pg/mL).
Further, the positive control (Dexamethasone)
treatment (G3) showed increased heart MIP-2 level by 40.6% i.e., 2438.50
± 255.71 pg/mL as compared to the G2 group. The level of MIP-2 was
decreased by 26.7% and 19.5% in the G6 (Cecal Slurry, LPS and E. coli along with Biofield Energy Treatment per se to animals from day -15) and G8 (Cecal Slurry, LPS and E. coli
along with Biofield Energy Treatment per se plus the Biofield Energy
Treated test formulation from day -15) groups, respectively as compared
to the untreated test formulation group (G4). The MIP-2 is a murine
counterpart of IL-8. MIP-2 is a naturally occurring inflammatory
cytokine biomarker in myocardium and its expression is increased during
myocarditis. Study reported that plasma MIP-2 levels are significantly
elevated in mice on days 7 and 14 of post-infection with
encephalomyocarditis (EMC) virus [40]. Taken together, our data suggest
that the Biofield Energy Treated test formulation and Biofield Energy
Treatment per se reduced the level of MIP-2 in heart tissues, which
could prevent the cardiovascular-inflammation.
Figure 6: The expression of heart macrophage
inflammatory protein-2 (MIP-2) in Sprague Dawley rats after treatment
with Biofield Treated test formulation and Biofield Energy treatment per
se to the animals. G1 as normal control (vehicle, 0.5% w/v CMC-Na); G2
as disease control (Cecal Slurry, LPS and E. coli + 0.5% CMC-Na); G3 as reference item (Cecal Slurry, LPS and E. coli + Dexamethasone); G4 includes Cecal Slurry, LPS and E. coli along with untreated test formulation; G5 as Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation; G6 group includes Cecal Slurry, LPS and E. coli along with Biofield Energy Treatment per se to animals from day -15; G7 as Cecal Slurry, LPS and E. coli + Biofield Energy Treated test formulation from day -15; G8 group includes Cecal Slurry, LPS and E. coli
+ Biofield Energy Treatment per se plus the Biofield Energy Treated
test formulation from day -15, and G9 group denoted Cecal Slurry, LPS
and E. coli along with Biofield Energy Treatment per se animals
plus the untreated test formulation. Values are presented as mean ± SEM
(n=6-9).
Estimation of Matrix Metallopeptidase-9 (MMP-9):
The expression of matrix metallopeptidase-9 (MMP-9) in heart tissue
after administration of the Biofield Treated/Blessed proprietary test
formulation and Biofield Energy Healing Treatment per se to the animals
was estimated, and the results are graphically presented in Figure 7.
The disease control (Cecal Slurry, LPS and E. coli + 0.5%
CMC-Na) group (G2) showed value of MMP-9 as 155.85 ± 12.62 pg/mL, which
was increased by 13.8% as compared with the normal control (G1, 136.96 ±
4.68 pg/mL). Further, the positive control (Dexamethasone) treatment
(G3) group decreased MMP-9 level by 8.1% i.e., 143.28 ± 7.66 pg/mL as
compared to the G2 group.
The level of MMP-9 was decreased by 6.4%, 9%, 2%, 15.8%, and 29.3% in the G5 (Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation); G6 (Cecal Slurry, LPS and E. coli along with Biofield Energy Treatment per se to animals from day -15); G7 (Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation from day -15); G8 (Cecal Slurry, LPS and E. coli
along with Biofield Energy Treatment per se plus the Biofield Energy
Treated test formulation from day -15), and G9 (Cecal Slurry, LPS and E. coli
along with Biofield Energy Treatment per se animals plus the untreated
test formulation) groups, respectively, as compared to the disease
control group (G2).
Besides, the level of MMP-9 was significantly reduced
by 12.6%, 15.1% (p≤0.001), 8.6%, 21.5% (p≤0.001), and 34% (p≤0.001) in
the G5, G6, G7, G8, and G9 groups, respectively with reference to
untreated test formulation (G4) group. MMP-9 is one of the most widely
investigated MMPs. MMP-9 expression has increases during cardiovascular
disorders like hypertension, atherosclerosis, and myocardial infarction.
MMP-9 degrades extracellular matrix proteins and activates cytokines
and chemokines to regulate pathological remodeling processes that
involve inflammation and fibrosis in cardiovascular disease [41].
According to one of the extensive research work done by Swedish
researchers, they found the high level of MMP-9 in coronary artery
disease (coronary artery ectasia) patients and a predictor of increased
mortality in that patients [42]. In this study, the Biofield Energy
Treated test formulation and Biofield Energy Treatment per se
significantly reduced the level of MMP-9, which could be beneficial to
combat inflammatory disease conditions in the cardiovascular patients.
Figure 7: The effect of the test formulation on the
level of heart macrophage inflammatory protein-2 (MIP-2) in Sprague
Dawley rats. G1 as normal control (vehicle, 0.5% w/v CMC-Na); G2 as
disease control (Cecal Slurry, LPS and E. coli + 0.5% CMC-Na); G3 as reference item (Cecal Slurry, LPS and E. coli + Dexamethasone); G4 includes Cecal Slurry, LPS and E. coli along with untreated test formulation; G5 as Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation; G6 group includes Cecal Slurry, LPS and E. coli along with Biofield Energy Treatment per se to animals from day -15; G7 as Cecal Slurry, LPS and E. coli + Biofield Energy Treated test formulation from day -15; G8 group includes Cecal Slurry, LPS and E. coli
+ Biofield Energy Treatment per se + Biofield Energy Treated test
formulation from day -15, and G9 group denoted Cecal Slurry, LPS and E. coli
+ Biofield Energy Treatment per se animals + untreated test
formulation. Values are presented as mean ± SEM (n=6-9). ***p≤0.001 vs.
G4. Experiment includes four preventive maintenance groups (G6, G7, G8
and G9). The findings showed the significant slowdown of
inflammation-related symptoms and also reduced the chances of disease
susceptibility. All-inclusive, it indicate that the Trivedi Effect® was
found to be most effective and benefited to protect different kinds of
diseases and also improve the overall health and quality of life.
Based on the study outcome it was found that the level of MPO was decreased by 12% in the G6 (Cecal Slurry, LPS and E. coli
along with Biofield Energy Treatment per se to animals from day -15)
group as compared to the untreated test formulation (G4) group.
Expression of SOD was significantly (p≤0.05) increased by 19.03%,
17.26%, and 11.81% in the G6, G7, and G9 groups, respectively as
compared to the G4 group. Moreover, the level of TNF-α was significantly
reduced by 25.97%, 40.28% (p≤0.01), 24.86%, 36.54% (p≤0.01), and 34.30%
in the G6, G7, G8, and G9 groups, respectively with reference to
disease control (G2) group. Additionally, IL-6 was significantly
(p≤0.001) decreased by 23.5%, 31.0%, 26.3%, and 39.8% in the G5, G6, G8,
and G9 groups, respectively as compared to the G2 group. Further, MIP-2
was decreased by 26.7% and 19.5% in the G6 and G8 groups, respectively
as compared to the G4 group. Besides, the level of MMP-9 was
significantly (p≤0.001) reduced by 15.1%, 21.5%, and 34% in the G6, G8,
and G9 groups, respectively as compared to the G4 group.
Altogether, the Biofield Energy Treated test
formulation and Biofield Energy Healing Treatment (the Trivedi Effect®)
per se showed significant results with respect to different inflammatory
biomarkers (cytokines) in the preventive maintenance group, G6 as well
as other preventive maintenance groups (G7, G8, and G9) in Cecal Slurry,
LPS and E. coli-induced systemic inflammatory response
syndrome model rat model study. It also helped to slowdown the
inflammatory disease progression and disease-related complications. The
study data showed that Biofield Energy Treated Test formulation and
Biofield Energy Treatment per se would be one of the best treatment
strategies to prevent the manifestation of diseases. Thus, the Biofield
Energy Treatment might act as a preventive maintenance therapy to
maintain and improve the overall health and quality of life and
simultaneously reduce the severity of acute/chronic diseases. The test
formulation can also be used against rheumatoid arthritis (RA), fibromyalgia, aplastic anaemia, Addison disease (AD),
multiple sclerosis, myasthenia gravis, psoriasis, Crohn’s disease,
ulcerative colitis, dermatitis, hepatitis, Parkinson’s, stroke, etc.
Click here: https://juniperpublishers.com/index.php