Gerontology & Geriatric Medicine - Juniper Publishers
Abstract
Objectives: A risk score for major toxicity related to non-steroidal anti-inflammatory drug (NSAID) use was recently introduced and validated in a very selected population. The objective herein was to test the feasibility of the calculator among community-dwelling middle-aged and older adults.
Methods: Questionnaire and clinical data of 2,644 citizens (mean age 64 years) were collected in the year 2002. NSAID use was determined as at least one purchase of prescribed NSAIDs during the considered year. The risk score was calculated based on the original introduced formula, except for hematocrit which was set into formula as the lowest and the highest reference values according to Finnish laboratory guidelines. A 10-year all-cause mortality of participants was examined.
Results: Of all participants, 25% had purchased prescribed NSAIDs. NSAID-related major toxicity risk distributions according to the score among participants were as follows: low risk 0.8%, intermediate risk 85 %, high risk 14% for low hematocrit value, and 17 %, 71% and 11% for high hematocrit value, respectively. The oldest participants were at the highest risk for major toxicity and had the highest NSAID purchasing percentage (29%). The higher risk score did not associate with mortality.
Conclusion: According to the score, majority of community-dwelling middle-aged and older adults carried at least intermediate risk related to NSAID use. Results herein did not support the usability of the risk score for major toxicity related to NSAID use in a population-based setting.
Keywords: Chronic pain; Elderly; NSAID; Analgesic; Chronic pain; Musculoskeletal pain; Adverse effects
Abbreviations: AKI: Acute Kidney Injury; ATC: Anatomical Therapeutic Chemical Classification System; BMI: Body Mass Index; CV: Cardiovascular; GOAL: Good Ageing in Lahti Region; NNH: Number Needed to Harm; NSAID: Non-Steroidal Anti-Inflammatory Drug; OA: Osteoarthritis; RA: Rheumatoid Arthritis; RCT: Randomized Controlled Trial
Introduction
NSAIDs comprise a major cause of drug-related morbidity [1-3]. Solomon and colleagues recently proposed a score for calculating a risk for major toxicity related to non-steroidal anti-inflammatory drug (NSAID) use [4]. A derivation and validation study of the score was done among NSAID users (median age 63 years) in a randomized controlled trial (RCT) setting in a selected cohort [4]. It was proposed that the score may be useful in clinical work. However, in order to put the score into use in clinics, it needs to be tested in less selected cohorts. Solomon and colleagues included several variables to predict major NSAID (celecoxib, naproxen, ibuprofen)-related adverse events: age, sex, history of cardiovascular disease, hypertension, diabetes mellitus, smoking, statin/lipid-lowering drug use, serum creatinine level, hematocrit level, rheumatoid (RA)/osteoarthritis (OA) [4]. Their study population consisted of patients with RA or OA and history of cardiovascular event, occlusive coronary or noncoronary arterial disease, diabetes mellitus, or other defined risk factors. Major toxicity was determined as occurrence of major cardiovascular (CV) events, acute kidney injury (AKI), significant gastrointestinal (GI) event, and mortality [4]. Therein, based on predicted one-year major toxicity risk probabilities, three risk groups emerged: low (<1%), intermediate (1-4 %) and high risk (> 4%) [4]. Proportion of participants that comprised each risk group were as follows: 4.6%, 68.6 % and 26.9%, respectively. Corresponding number-needed to harm (NNH) was 100 for low risk and 25 for high risk. A properly validated risk score calculator could prove useful in clinical decision making, and possibly lead to health-related benefits. Solomon and colleagues preliminarily validated the risk calculator in patients with increased risk for NSAID –related adverse effects. However, NSAIDs may cause marked harm for individuals without known or with latent risk factors. Thereby, if proved operative, such risk calculator could provide an important tool for risk estimation especially in general patient material, whose NSAID –related risks would possibly otherwise not be recognized. The purpose of the current study was to test the feasibility of the previously presented NSAID -related major toxicity risk score among community-dwelling citizens with diverse medical backgrounds.
Materials and Methods
The current study used data from a 10-year follow-up Good Ageing in Lahti Region (GOAL, year 2002-2012) cohort study executed in Lahti Hospital District in Southern Finland [5]. The GOAL introduced a stratified (age, sex, 14 municipalities) random sample of Finnish aging adults born in 1926-30, 1936-40, and 1946-50. The present study focused on the baseline year 2002 data; thus, the participants were 52-56, 62-66 and 72-76 years of age. A total of 4,272 randomly selected community-dwelling participants were invited, of which 2,815 (66%) attended. Data regarding 2,644 participants with sufficient NSAID-data were included in the current study. Data regarding NSAID purchases were retrieved from the Social Insurance Institution of Finland (SII), that maintains a nationwide register of all prescriptions and medication purchases. NSAID use was determined as at least one purchased package of prescribed NSAIDs during the year 2002. Classified with the Anatomical Therapeutic Chemical Classification System (ATC), the NSAIDs included were: M01AE01, M01AE51 ibuprofen; M01AE03 ketoprofen; M01AH01, M01AH05, M01AH06 COX-2 selective inhibitors; M01AC06 meloxicam; M01AE02, M01AE52 naproxen; M01AB05 diclofenac and M01AB01 indomethacin. Acetylsalicylic acid was excluded from the NSAIDs due to its major use as an antithrombotic drug. Additionally, an extensive questionnaire (overall health, attitudes, quality of life, etc.) data, blood samples and physical data were collected. Data regarding baseline characteristics were based on the questionnaire. Variables included age, sex, smoking, cardiovascular disease, hypertension, diabetes mellitus, statin/lipid-lowering drug use, serum creatinine level, rheumatoid (RA)/osteoarthritis (OA) and Body Mass Index (BMI). The risk scores were calculated based on the original formula [4]. All needed variables were retrievable except for hematocrit level. Therefore, hematocrit was set into formula as the lowest and the highest reference values according to Finnish laboratory guidelines: 35-46 % for females, 39-50 % for males. With administered variables inserted into the formula, the risk scores were calculated as follows: (0.0325 x age) + (0.2666 x sex [male=1]) + (0.8352 x cardiovascular disease [yes=1]) + (0.2252 x hypertension [yes = 1]) + 0.3434 x diabetes mellitus [yes=1]) + (0.3653 x smoking [yes = 1]) + (0.1849 x statin/lipid-lowering drug use [yes=1]) + (1.0964 x serum creatinine [per 1mg/dl increase]) + (0.5403 x rheumatoid arthritis [yes=1])–(0.0742 x hematocrit reference value). The 10-year mortality of participants was examined. Mortality data were retrieved from Statistics Finland. The Regional Ethics Committee of Tampere University Hospital gave the approval for the study. The principles of the Declaration of Helsinki were observed. All participants gave their written informed consent prior to the data collection.
Results
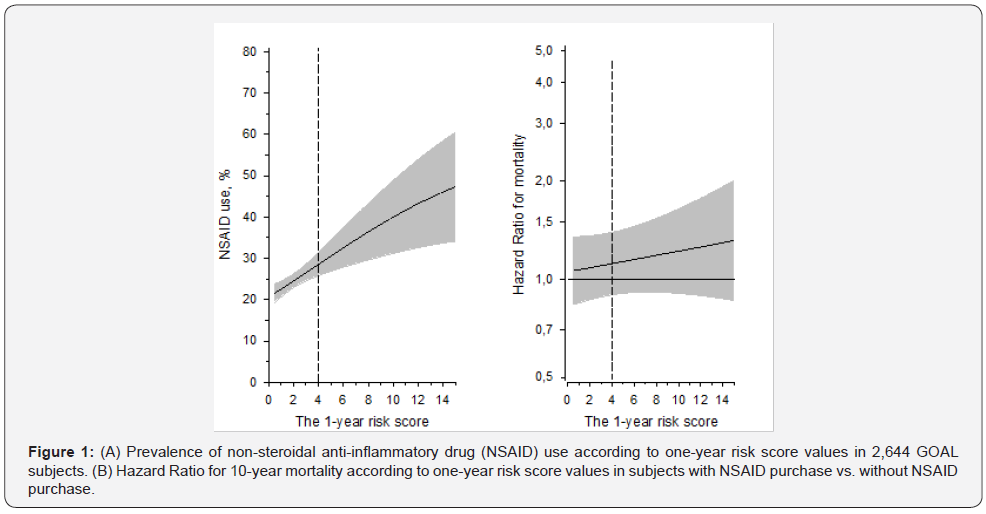
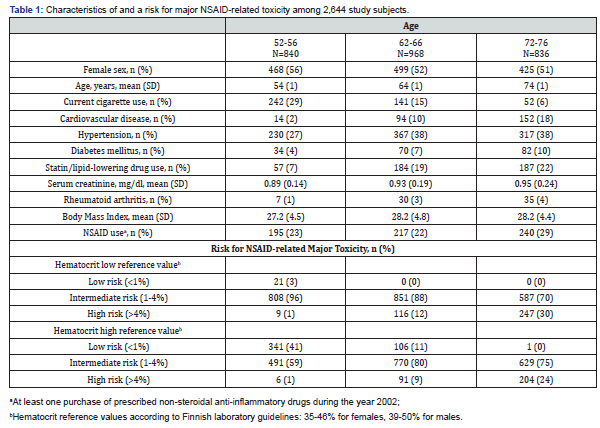
Mean age of all 2,644 participants was 64 years. 1,392 of 2,644 were females (53%). One of ten had known cardiovascular disease. 652/2,644 (25%) had purchased prescribed NSAIDs during the year 2002. NSAIDs were to the highest extent (29%) purchased by the oldest participants (72-76 years). Participant characteristics and baseline data are presented in (Table 1). For the lowest hematocrit reference value, NSAID-related major toxicity risk distribution in the whole sample was as follows: low risk 0.8%, intermediate risk 85 %, high risk 14%. For the highest hematocrit reference value, the risk distribution was: 17 %, 71% and 11%, respectively. According to the score, the oldest participants were at the highest risk for major toxicity. NSAID purchases according to one-year risk score values in 2,644 GOAL subjects are presented in (Figure 1A). NSAIDs were to the highest extent used by participants with high risk for major toxicity. Hazard Ratio for real world 10-year mortality according to one-year risk score values in subjects with NSAID purchase vs. without NSAID purchase are presented in (Figure 1B). The higher risk score did not markedly associate with the increased mortality risk.
Discussion
The purpose of this study was to examine feasibility of previously introduced NSAID-related major toxicity risk score in a general aging population [4]. The results did not support the sensibleness of utilization of the calculator in community-dwelling adults. NSAIDs are commonly used analgesics, also among aging population [6,7]. However, several NSAID-related adverse effects have been reported, e.g., gastrointestinal ulcers, cardiovascular events and renal adverse effects [1-3,8,9]. Major toxicities may occur without an obvious underlying risk factor. According to the toxicity risk calculated herein, NSAID administration would only appear to be regarded as safe for less than one percent of community-dwelling aging adults with hematocrit at its lowest reference value. With high hematocrit level could NSAIDs be safely used for less than one fifth of middle-aged and older adults. Thus, according to the score, for majority of the aging population, NSAIDs would be forbidden. This could cause major difficulties in clinics. Musculoskeletal conditions are common in advancing age [10], and for these conditions NSAIDs are effective [11]. For many patients, pro re nata NSAID use provides adequate pain relief. If the recommendations of the toxicity risk score were followed, NSAIDs would likely be needed to be replaced with other analgesics that are effective for nociceptive pain, e.g., opioids or serotonin-noradrenaline reuptake inhibitors (not possible to use pro re nata), which also carry a risk for several adverse effects [12,13]. According to the results, in this population, NSAIDs were to the highest extent used by participants with the highest risk score. In addition to elevated risk for other serious hazards, it is well known, that chronic NSAID use may increase mortality [14]. However, according to our population-based data the documented NSAID use in this elderly series did not associate with significantly elevated mortality risk during 10-year follow-up between participants with low, intermediate or high risk for major toxicity. Mortality may be regarded as an endpoint variable also for other major hazards, that were not considered herein.
Thus, in clinical work, the risk of NSAIDs should not be overestimated if predicted benefit of the analgesic treatment may be estimated as significant. Effective pain medication probably enables activities that maintain physical condition and, therefore, may also decrease at least the mortality risk. Therefore, selection of pain treatment modality and analgesic should always be evaluated individually based on type of pain and patient-related factors. It would be important to have a score which could help clinicians to evaluate the true risks of pharmacological treatment. However, risk score supplied by the calculator presented by Solomon and colleagues did not emerge as all solid in a population-based setting. It is possible, that the presented calculator would provide misleading results in clinical administration. The limitations of this study include lack of individual hematocrit values. However, analyses were calculated with laboratory reference values, that provide the highest and lowest extremity risks for major toxicities. Second, the exact amount of NSAIDs used was not retrievable, as NSAID use was only determined as at least one purchased NSAID prescription per year. However, it is possible that even a higher number of participants had actually used NSAIDs than what presented herein, when combining NSAIDs obtained over the counter [15]. An importance of future studies with long-term follow-up regarding NSAID related hazards needs to be underlined.
Conclusion
We conclude that the score proposed by Solomon and colleagues may prove helpful in noticing the risk factors of NSAID toxicity in selected cohorts, however, on population level it seems to evaluate the risk much higher than appropriate. Thus, it is possible that the score would limit the use of NSAIDs unnecessarily and cause controversies in the selection of optimal medical treatment. We are still looking forward to getting a tool to evaluate the risk of NSAIDs in diverse clinical situations.
To Know more about Gerontology & Geriatric Medicine
Click here: https://juniperpublishers.com/index.php




No comments:
Post a Comment