Organic & Medicinal Chemistry - Juniper Publishers
Abstract
Skin is the largest organ of the human body with the principal role of first barrier from external stimuli. Exposure to ultraviolet-B (UV-B) radiations and urban pollutants trigger the rapid generation and accumulation of reactive oxygen species (ROS) in skin cells, with the consequent increase in oxidative stress. The development of cosmetics rich in antioxidant compounds is an important strategy to prevent skin-ageing. To substantiate the relative claim, we develop a panel of chemical and cell-based methods to use on a cosmetic formulation, as the ANTIOX SERUM, enriched with functional compounds known to counteract ROS and consequently oxidative stress-related skin-ageing. The antioxidant property of the product was firstly evaluated with the DPPH and Folin-Ciocalteu tests. Using a human keratinocyte cell line, the protective effect against UVB-induced cellular oxidative damage was performed with the DCF-DA assay and the measurement of the ROS levels. The antipollution activity was also investigated using the same experimental method using the benzo[a]pyrene as a stimulus. The obtained results confirm the antioxidant efficacy of the cosmetic product through preliminary chemical screening tests. In HaCaT cells, the cosmetic formulation showed a statistically significant ROS levels reduction after 24 h of treatment with the non-cytotoxic concentrations of the ANTIOX SERUM both in the antioxidant and antipollution in vitro models. Our findings confirmed that the combination of chemical and cell-based antioxidant methods are useful to substantiate the antioxidant and antipollution claims of the cosmetic formulation enriched with functional molecules, such as coenzyme Q10 (ubiquinone), α-lipoic acid (thioctic acid), 3-O-etil-L-ascorbic acid and glutathione.
Keywords: HaCaT cells; Oxidative stress; Antioxidant; UVB radiation
Abbreviations: PAHs: Polycyclic Aromatic Hydrocarbons; ECM: Extracellular Matrix; UVB: Ultraviolet-B; HaCaT: Human Keratinocyte Cell; MMP-1: Metalloproteinase-1; ROS: Reactive Oxygen Species; SD: Standard Deviation; PBS: Phosphate Buffer Saline; OD: Optical Density; FCR: Folin-Ciocalteu Reagent; DHLA: Dihydrolipoic Acid; INCI: International Nomenclature of Cosmetic Ingredients; UV: Ultraviolet
Introduction
Skin is the largest organ of the human body and the first protective barrier from the external stimulus; influencing people's social behaviour through a more youthful and healthy appearance, it presents also an important cosmetic role [1]. Since skin functionality can be compromised by intrinsic and extrinsic factors, finding a way to preserve its integrity is always a current challenge. The first mentioned “intrinsic factor” is referred to the physiological and chronological processes of decay due to genetic factors, which tends to begin after 25 years of age and results in thin, dry skin and fine wrinkles. Extrinsic ageing is engendered by the aggression of external environmental factors as air pollution, smoke and sun exposure that result in coarse wrinkles, loss of elasticity, laxity and rough-textured appearance [2]. In particular, long-term exposure to solar ultraviolet (UV) radiation is the primary cause of extrinsic skin ageing and it is referred to as photo-ageing [3]. UV radiation is divided into three sub-categories based on the wavelength (UVA, UVB and UVC) and it is responsible for about 80% facial ageing through an increase of reactive oxygen species (ROS) and oxidative stress [4]. Both of them can be counteracted by the antioxidant defence system produced by the skin [5]. Antioxidants are chemically stable molecules in the reduced form, capable of yielding electrons or hydrogens to compounds with unpaired electrons to stop the propagation of radical processes. However, when the damage is very extensive, the intrinsic mechanisms are often not enough [6]. For this reason, exogenous antioxidants are used in cosmetic products to support the commercial claim of anti-ageing [7]. The antioxidant property is mainly correlated with the contents of polyphenols, proteins/peptides, vitamins C and E, and a few metals such as selenium [8]. The proposed experimental design was developed using a cosmetic serum, called ANTIOX SERUM, (Matex Lab, Brindisi, Italy), an enriched formulation with four bioactive compounds within the International Nomenclature of Cosmetic Ingredients (INCI), known for their ability to counteract skin ageing: coenzyme Q10, α-lipoic acid, 3-O-etil-L-ascorbic acid and glutathione. Coenzyme Q10, or Ubiquinone, in its reduced form, can release a hydrogen from the benzene group blocking the radical and consequently the oxidative reaction. Furthermore, Coenzyme Q10 exhibits antioxidant activity with a key role in mitochondrial respiration [9]. Even α-lipoic acid, or Thioctic Acid, acts in the reduced form dihydrolipoic acid (DHLA) which is able to limit the development of radicals but also to restore the inactive forms of other antioxidants [10,11]. The 3-O-etil-L-ascorbic acid derives from ascorbic acid, with the addition of an ethyl group on the hydroxyl: this new structure leads to greater protection against ionization of the OH group with the consequent block of oxidation. The molecular modification allows improving the antioxidant activity compared to ascorbic acid, especially in areas exposed to the air such as the skin [12]. Glutathione is one of the main molecules that protect the skin from UV radiation thanks to the activity of the enzyme glutathione peroxidase, which catalyzes the elimination reaction of hydrogen peroxide inside the cell cytoplasm [10]. δ-tocopherol is an isomer of vitamin E, which acts by transferring an hydrogen of the hydroxyl group which blocks O21 and other ROS [11]. The objective of this study is to develop a panel of in vitro methods that can be used to support the antioxidant and antipollution capacities of a selected cosmetic formulation containing functional ingredients. At first, we investigated the free radical scavenging and the phenol content activities using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and Folin-Ciocalteu methods, respectively. Moreover, the antioxidant and antipollution activities have been evaluated using an immortalized cell line of keratinocytes stimulated with UVB and urban pollutant, respectively.
Materials and Methods
Materials
Ascorbic acid (code A4403, Merck, Darmstadt, Germany), 2,2-diphenyl-1-picrylhydrazyl (DPPH, code 44150, Thermo Fisher Scientific, Waltham, MA, USA), benzo[a]pyrene (B[a]P, B1760, Merck, Darmstadt, Germany), 2’,7’-dichlorodihydrofluorescein diacetate (H2DCF-DA, D6883, Merck, Darmstadt, Germany), ethanol (Et-OH, code 02860-1L, Honeywell, Charlotte, NC, USA), High Glucose Dulbecco’s Modified Eagle’s Medium (DMEM, code BE12-614F, Euroclone, Milan, Italy), Fetal Bovine Serum (FBS, code ECS0180D, Euroclone, Milan, Italy), Folin-Ciocalteu Reagent (code 1.09001.0100, Merck, Darmstadt, Germany), L-glutamine (code ECB3000, Euroclone, Milan, Italy) antibiotics (107 Units/l penicillin G sodium and 10000 mg/l streptomycin sulfate, code ECB3002D, Euroclone, Milan, Italy), and Sodium Carbonate (Na2CO3, code 0246, VWR International, Milan, Italy).
INCI ingredients
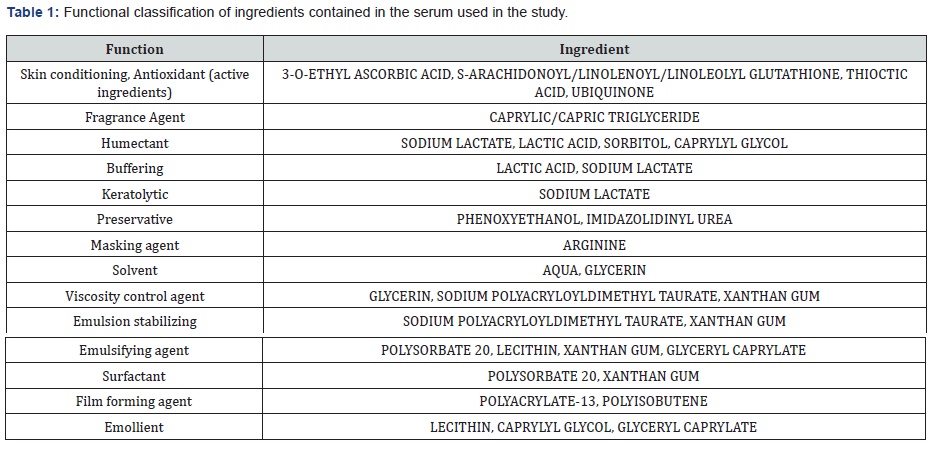
Based on the INCI components, the cosmetic formulation enriched with antioxidant ingredients (Table 1) was taken under investigation.
Cell-free chemical antioxidant assays
Sample preparation: The cosmetic formulation was prepared by making 10% w/v solution in a 70:30 EtOH:H2O, mixed and incubated in the dark for 30 min. Samples were centrifuged at 4000 rpm for 10 min (Mega Star 600R, VWR International, Milan, Italy) and after, supernatants were preserved until use.
DPPH and folin-ciocalteu tests
The radical scavenging potential of the cosmetic product was measured using the DPPH assay. Based on the principles described by Mukesh Singh in 2017 [13], a stock solution of 0.6 mM DPPH was prepared and left in the dark for 2 h. Then, 2 mL of DPPH solution was added to 1 mL of the sample. After 30 min incubation, the absorbance was read at 517 nm (Multiskan Go, Thermo Fisher Scientific, Waltham, MA, USA). The polyphenolic content of samples was determined by the Folin-Ciocalteu method using the mixture of sodium tungstate and sodium molybdate (Folin-Ciocalteu reagent), which is able to oxidize antioxidant molecules and polyphenolic substrates. One hundred µL of the sample were mixed with 1 mL H2O and 0.5 mL of Folin-Ciocalteu reagent (FCR). The solution was vortexed and 1 mL of Na2CO3 20% w/v solution was added to each sample. After 1 h at 95 °C, the absorbance was read at 765 nm (Multiskan Go, Thermo Fisher Scientific, Waltham, MA, USA). A qualitative assessment was also performed for both methods. Internal controls, represented by ascorbic acid and a cosmetic product proved to be without antioxidant activity (CTRL-), were also included.
Cell-based antioxidant assays
Cell culture: HaCaT cells (code BSCL168, Zooprof. Institute of Lombardy e Emilia Romagna, Brescia, Italy) were grown in a complete medium constituted by DMEM containing 10% FBS and 1% antibiotics and L-Glutamine and in conditions of complete sterility in incubation at 37°C with 5% carbon dioxide (CO2) atmosphere.
Cytotoxicity assay (MTT test): As a preliminary assay, the cytotoxicity was evaluated following the method describes by Mosmann [14]. HaCaT cells were homogeneously seeded into 96-well plates at a density of 2 x 104 cells per well. After 24 h, cells were treated with different concentrations of the cosmetic formulation, starting from 40 mg/mL, prepared directly in a culture medium and following serial dilution (1:2). Untreated cells were used as control. After 24 h of treatment, the culture medium was then removed and cells were incubated with 50 µL of MTT solution (1 mg/mL) at 37 °C for 2 h. Subsequently, the solution was removed and replaced with 100 µL of isopropanol. Absorbance was determined at 560 nm wavelength using a microplate reader (GloMAx®, Promega Corporation, USA). Cell survival was calculated by measuring the difference in optical density (OD) of the tested cosmetic formulation with respect to control (untreated cells).
Cell viability (%) = [OD570nm tested product/OD570nm control] x 100
Measurement of intracellular ROS: Intracellular ROS levels were measured using the fluorescent probe H2DCF-DA, as described by Boulton and colleagues [15]. Briefly, HaCaT cells were seeded in a 96-black well plate at 37 °C with 5% CO2. After 24 h, cells were treated for a subsequent 24 h with the tested product, choosing the two highest non-cytotoxic concentrations (5 and 10 mg/mL) with the best solubility, selected by the preliminary MTT assay. Ascorbic acid was also included as an internal control being a known molecule able to protect from free radical damage. At the end of the treatment, HaCaT cells were washed with PBS and incubated with 20 mM DCF-DA solution at 37 °C with 5% CO2. Cells were then washed with phosphate buffer saline (PBS) and exposed to UVB lamp (CAMAG® UV Lamp 4, wavelength 302 nm, Muttenz, CH) with 2.5 mJ/cm2 dose. At the end, HaCaT cells were analysed using a fluorimeter (GloMAx®, Promega Corporation, USA) with excitation of 475 nm and emission at 500-550 nm. Relative ROS production was expressed as the ratio of fluorescence of the treated samples over the response in the appropriate controls:
(fluorescence treatment/fluorescence control) × 100.
Antipollution activity (ROS reduction): The antipollution activity of the cosmetic formulation was evaluated through its ability to decrease ROS formation in HaCaT cells after stimulation with a known pollutant (B[a]P) followed by DCF-DA protocol, as previously described. HaCaT cells were seeded in a 96-well black plate at 37 °C with 0.5% CO2. After 24 h, cells were co-treated for 24 h with the pollutant (B[a]P) and with the product at the two non-cytotoxic concentrations (5 and 10 mg/mL). Untreated cells were used as control. Cells treated with B[a]P were used as positive control while treatment with ascorbic acid was used to confirm the reduction of the oxidative stress in the cellular environment. At the end of the treatment, HaCaT cells were washed with PBS and incubated with the DCF-DA solution as previously described.
Statistical analysis
Data are presented as mean ± standard deviation (SD) of at least three independent experiments performed in duplicate. Statistical significance was calculated in all experimental sets using the One-way ANOVA with Bonferroni’s LSD multiple comparisons as post-test. p < 0.05 was considered a statistically significant difference compared to the relative controls. Statistical analysis was performed using GraphPad Prism version 9.0.0 (GraphPad Software, San Diego, CA).
Results
Radical scavenging potential by DPPH and Folin-Ciocalteu tests
The DPPH and Folin-Ciocalteau tests are used to determine the antioxidant activity and the total contents of polyphenols and ascorbic acid, respectively, directly performed on the finished cosmetic formulation. Table 2 shows the spectrophotometric results, expressed as OD (mean ± SD). In the DPPH experiments, the OD measured for the cosmetic formulation was equal to 0.265 and it was very close to the OD obtained with the ascorbic acid (0.104). It was possible to note that after adding the DPPH reagent, the purple colour was turned to yellow, whereas no change colour was instead observed in the cosmetic product without antioxidant activity, used as negative control (CTRL -) (Figure 1A). In the Folin-Ciocalteau method, the OD measured for the cosmetic formulation and the ascorbic acid were equal to 0.797 and 1.527, respectively, together with the visible blue colour of the samples. Unaltered colour, correlated to the absence of phenolic content, was observed, as expected, in the negative control (Figure 1B).

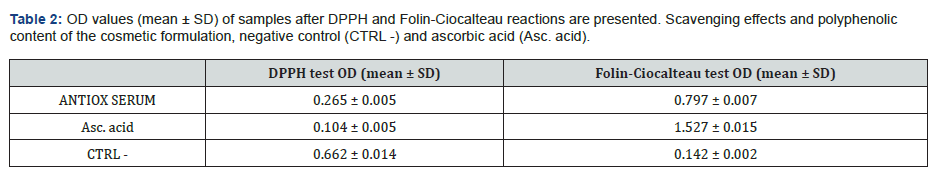
Evaluation of the cytotoxicity by MTT assay
The cytotoxicity test is performed only on the cosmetic formulation, the ANTIOX SERUM, demonstrated to have an antioxidant activity by the DPPH and Folin-Ciocalteu as preliminary assays. To evaluate the cell viability and to identify the appropriate concentrations, an MTT test was performed. Figure 2 shows the results of HaCaT viability after 24 h treatment (range tested 0.313 to 40 mg/mL for 24 h) with the cosmetic formulation under examination. The obtained data demonstrated cytotoxic activity only with the highest concentrations tested equal to 20 and 40 mg/mL with a percentage of cell viability of 69.82 and 28.15%, respectively, compared to untreated cells. Therefore, 5 and 10 mg/mL were selected for further in vitro studies.
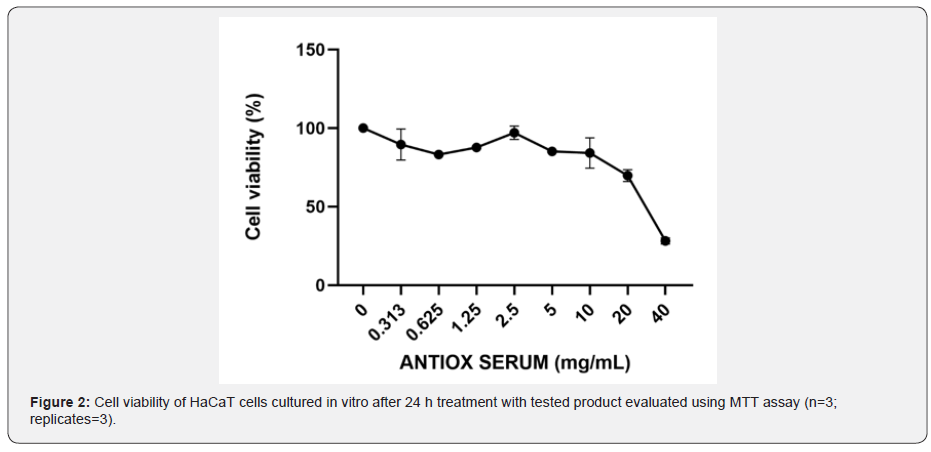
Evaluation of antioxidant activity after UVB radiation
To investigate the protective antioxidant effects of the cosmetic formulation in UVB-irradiated HaCaT cells, the intracellular ROS levels were evaluated using a fluorescent probe H2DCF-DA. As shown in Figure 3, UVB significantly increased the ROS levels compared with untreated cells (p ˂ 0.0001). In the presence of UVB, the cosmetic formulation significantly reduced the ROS level in a concentration-dependant manner. Particularly, it is possible to observe a highly significant reduction (p ˂ 0.0001) of 19.23 and 23.89% after treatment with 5 and 10 mg/mL, respectively. Moreover, ascorbic acid was confirmed as good internal control, capable to reduce ROS levels of about 20%.
Evaluation of antipollution activity
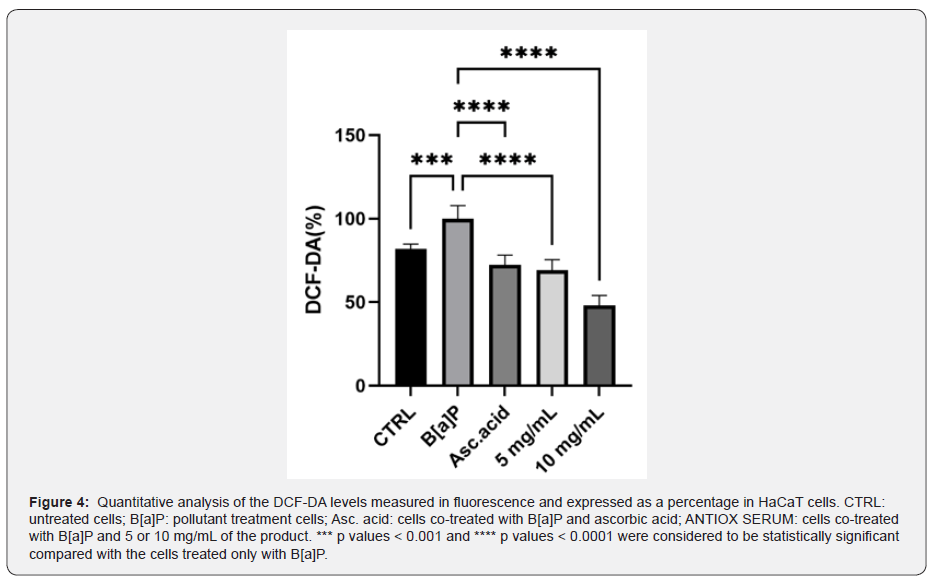
To investigate the potential antipollution effect of the cosmetic formulation, the intracellular ROS levels were evaluated using a fluorescent probe H2DCF-DA in HaCaT cells treated with B[a]P. As shown in Figure 4, cells treated with B[a]P significantly increased the ROS generation compared with those in the untreated cells (p ˂ 0.001). Therefore, we evaluated the effect of the product after 24 h treatment in the presence of B[a]P, used as an urban pollutant. It can be seen that the treatment of the tested product at both concentrations selected led to a strong reduction in ROS levels when compared with HaCaT cells treated only with the pollutant B[a]P: 26.81 and 42.39% less with 5 and 10 mg/mL, respectively (p ˂ 0.0001). Ascorbic acid is confirmed to be a good positive control, which reduces the ROS levels of about 23%.
Discussion
The skin is the first external barrier of our body and it is heavily exposed to environmental stressors. All of them act directly on skin cells through the generation of free radicals and especially reactive oxygen species (ROS) with the consequent stimulation of skin ageing-related signalling pathways, as the matrix metalloproteinase-1 (MMP-1) [16]. Clinically, the biological consequences of an overloading amount of free radicals include wrinkling, laxity, and a leather-like appearance [17]. To counteract the adverse changes induced by ROS levels, the skin is equipped with an effective defence mechanism, consisting of an interacting network of antioxidants, that can be further improved with the daily use of cosmetic products containing functional antioxidant compounds [7,17]. The use of in vitro methods to highlight the antioxidant and antipollution activities is therefore a precise approach to support the relative claims. Currently, many analytical methods allow the determination of the antioxidants’ levels in plant materials and foods [18], but not all of them are appropriate for cosmetic products, which are a mixture of compounds with different physicochemical properties [19]. In our work, we want to present a panel of in vitro tests useful to demonstrate the antioxidant activity of the selected cosmetic formulation, the ANTIOX SERUM, produced by MatexLab (Brindisi, Italy) containing coenzyme Q10, α-lipoic acid, 3-O-etil-L-ascorbic acid and glutathione. Therefore, we decided to perform chemical and cell-based assays making a comparison with a cosmetic product without antioxidant activity. Among the chemical ones, the DPPH and Folin-Ciolcalteu assays were carried out, obtaining a good antioxidant activity for only the ANTIOX SERUM, as expected. The use of these chemical assays provides preliminary data that could be used as screening, considering that both methods are simple, efficient and relatively inexpensive. These assays, however, do not consider the cellular mechanisms. Thus, to better assess the antioxidant capacity of the only ANTIOX SERUM in a more physiological way, experiments were performed in a cellular setting using human keratinocyte cell lines (HaCaT) and ultraviolet-B (UVB) lamp as the irradiation source.
UVB-irradiation is indeed one of the main causes of increased ROS, capable to induce MMPs expression and the degradation of collagen and elastin fibers, the main structural proteins of the dermal extracellular matrix (ECM) [16,20,21]. These sets of skin alterations could contribute to photo-ageing development. Our results demonstrated the protective effect of the cosmetic formulation against UVB irradiation, with the ROS levels reduced of about 20% after 24 h treatment with the two non-cytotoxic concentrations (5 and 10 mg/mL) in HaCaT cells. We then wondered whether the cosmetic formulation could also have the ability to protect against pollutants. It is well known that environmental pollutants contribute to the increase of oxidative stress and premature skin ageing and the benzo[a]pyrene is one of the most important investigated [22]. It belongs to the polycyclic aromatic hydrocarbons (PAHs), capable to induce the increase in ROS levels in different cell lines, including HaCaT cells [23,24]. In our experimental model, the cosmetic formulation showed a strong ROS level reduction, of about 27% and 42% after treatment with 5 and 10 mg/mL, respectively. Overall, our findings confirmed the good antioxidant activity and the discovery an antipollution effect of the ANTIOX SERUM that can be used to support the relative claims.
The results of our study show an experimental design, composed of chemical and cell-based assay, to prove and support the antioxidant activity of different cosmetic formulations, as demonstrated by testing the product ANTIOX SERUM. Chemical assays have the significant advantage of requiring less time, instrumentation readily available in any laboratory and providing a reliable preliminary analysis. The cellular experiments permit not only to confirm the antioxidant activity in a living environment such as cell cultures but also to find out an antipollution effect that can be used to substantiate the cosmetic product claim.
To Know more about Organic & Medicinal Chemistry
Click here: https://juniperpublishers.com/index.php




No comments:
Post a Comment