Pharmacy & Pharmaceutical Sciences - Juniper Publishers
Abstract
Objective: The aim of this study is to determine the applicability of UV detection method by the sensitive, simply, precise, specific, and low-cost High-Performance Liquid Chromatography (UHPLC) for acetylsalicylic acid in presence of its degradation product.
Methods: Method development and determination of dissolution rate for acetylsalicylic acid, were achieved by using reversed-phase liquid chromatography with PDA (Photodiode Array Detector) and TUV (Tunable UV Detector) detection by HCLASS-UHPLC. The new proposed method utilized by the Waters Acquity UHPLC® TUV and PDA systems using a UHPLC column Waters Acquity, BEH, C18, 2.1mm x 50mm, 1.7μm particle size with a mixture of heptane-1-sulphonic acid sodium salt solution which was prepared in acetonitrile: deionized water (15%: 85% v/v) in isocritic mode at a flow rate of 0.5ml/ min, at 25°C with a load of 5μL. The detection for all eluted compounds was carried out at 280nm.
Results: The amount of acetylsalicylic acid’s active metabolite [1] salicylic acid (Figure 1) can also be determined in the same chromatographic conditions and resolution between Acetylsalicylic acid and salicylic acid is more than 2.0. The chromatographic parameters such as retention times, resolution, peak symmetry and theoretical plate count were determined. The validation studies of the developed method were conducted as to be perform the parameters specificity (resolution; 4.0, purity angle < purity threshold), linearity (r > 0.99), system precision, (RSD: 0.06% - 0.12%), repeatability (RSD: 1.69%), intermediate precision (RSD: 0.94%), accuracy and robustness (96.6% - 98.% for Acetylsalicylic Acid, 95.6% - 98.9% for Salicylic Acid) specified in the ICH (The International Conference on Harmonization) Q2(R1) [2]. Guideline and the results meet the acceptance criteria [3].
Conclusion: The developed method proved that the results obtained from the determination of dissolution rate in enteric-coated tablets containing acetylsalicylic acid are repeatable and sensitive. Also found suitable for the analysis of the stability samples of the drug product.
Keywords: Acetylsalicylic acid development; Dissolution; LC; Validation
Abbreviations: USP: United States Pharmacopoeia; LC: Liquid Chromatography; ACN: Acetonitrile; QSM: Quaternary Solvent Manager
Introduction
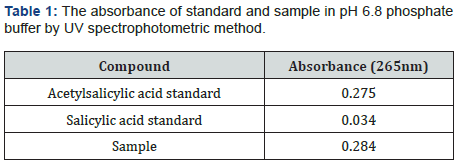
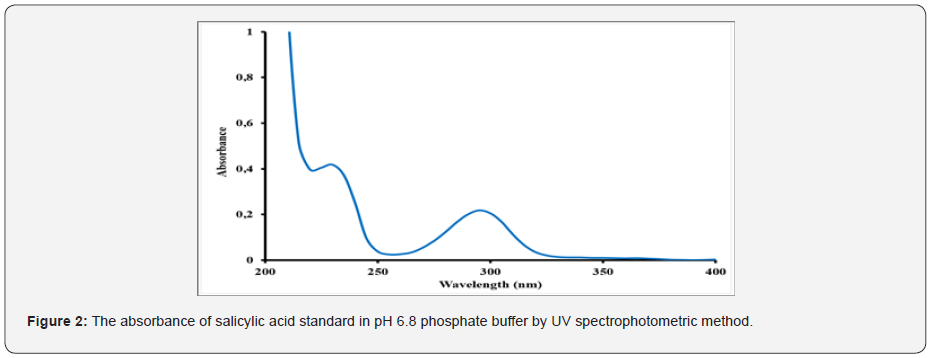
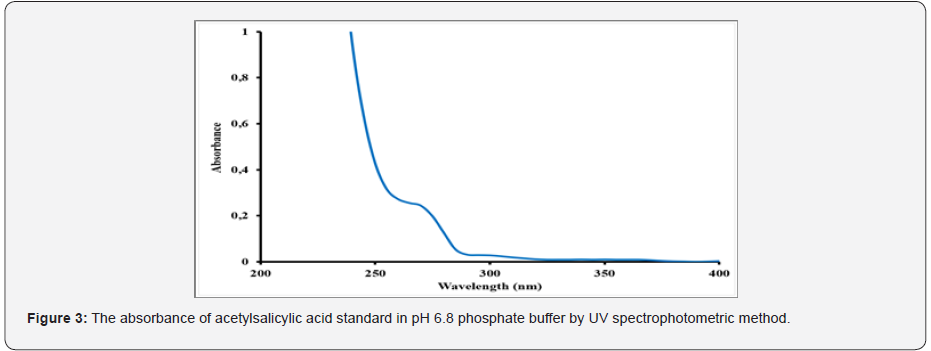
Acetylsalicylic acid, also known as an active substance of aspirin, is used an antipyretic analgesic for over a century. This widely known and accepted medicine can be used to treat wide range of diseases such as colds, fever, headaches, toothache, joint pain, and rheumatism. Aspirin can also inhibit platelet aggregation, prevent, and treat ischemic heart disease, cardiopulmonary infarction, cerebral thrombosis, and other diseases [4]. Moreover, it has shown that acetylsalicylic acid has the anti-cancer effect [5]. The US working group for preventive services has issued a guide that acetylsalicylic acid can be used as the primary prevention of cardiovascular disease and colorectal cancer. The guideline proposed for the first time that high-risk groups of non- colorectal cancer could use acetylsalicylic acid for the primary prevention of colorectal cancer [6]. It indicated that taking a certain dose of acetylsalicylic acid daily might effectively block the growth of breast cancer [7]. Additional studies are investigating the potential of acetylsalicylic acid to boost the immune system, treat cognitive decline and lower the risk of colon and ovarian cancer. A low daily dose, 75-81mg, is generally used in the preventive dose of acetylsalicylic acid therapy. Commonly, acetylsalicylic acid has been regarded as a potential gastric irritant [8] and studies have shown that the occurrence of gastric intestinal side effects might increase with regular use of the drug [9]. Enteric coating of the tablets is helpful for preventing stomach upset or irritation in those taking the daily dose of acetylsalicylic acid treatment. Due to the moisture-sensitive nature of aspirin drug, it can hydrolyze into acetic and salicylic acids when it is contacted to air with high humidity and elevated temperatures [10]. As the coating process will expose acetylsalicylic acid tablets to both high temperatures and humidity, it is significant that the formulation is resistant to moisture interaction during this process. The ideal enteric-coated tablets need to show perfect stability under accelerated storage conditions without the use of extra (and more expensive) packaging precautions such as desiccant packages or other specialized packaging materials [11]. Monographs of different forms of the official aspirin are available in USP, Polish Pharmacopoeia V, British Pharmacopoeia [12,13]. There are several analytical methods to assess aspirin product quality and batch to batch consistency of the production. One of the main methods is a dissolution performance test and salicylic acid determination. Dissolution test is used to obtain dissolution time and amount of dissolved active substances. Salicylic acid is an active metabolite of acetylsalicylic acid. The maximum allowed percentage for free salicylic acid in Aspirin Extended-Release Tablets and Aspirin Delayed-Release Tablets are 3% and in ‘Aspirin Effervescent Tablets for Oral Solutions’ is 8% [12]. Different methods have been reported for the determination of aspirin and salicylic acid [12-17] and USP 23 procedure includes HPLC analysis of the free salicylic acid. American Pharmacopoeia (USP) is often used as a reference in finished product testing applications. The specific dissolution technique employed is determined by the dosage form characteristics and the intended route of administration. For solid dosage forms, industry standard dissolution testing methodologies are the United States Pharmacopoeia (USP) Apparatus 1 (basket) and the USP Apparatus 2 (paddle). Floating capsules and tablets generally use USP 1 baskets [18]. According to monograph in the US Pharmacopoeia 39 (USP 39) [19] regarding tablet form containing the acetylsalicylic acid active substance, the method of dissolution rate determination is the UV spectrophotometric method. It was defined in the dissolution rate studies conducted according to this method that acetylsalicylic acid turns into its active metabolite salicylic acid in pH 6.8 phosphate buffer, Stage 2 and it could not be detected separately by applying UV spectrophotometric method (Table 1) and (Figure 2-4). The simultaneous evaluation of dissolution test of active substance acetylsalicylic acid and active metabolite salicylic acid is not possible by direct UV spectrophotometry. Therefore, it was decided to develop a more specific method by applying Liquid chromatography (LC) to determine both acetylsalicylic acid and its active metabolite salicylic acid in a short time. In this study, a new liquid chromatography (LC) method, which is more selective than UV spectrophotometric method used in determining the dissolution rate of enteric coated tablets containing acetylsalicylic acid was developed and validation study was performed. The significant number of method trials are performed to obtain the best results. The duration of the analysis was shortened but it was decided to switch to the faster response HPLC system to prevent the degradation of acetylsalicylic acid.

Experimental
Chemicals and reagents
The chemicals that are used in the study were of analytical reagent grade. Acetonitrile (ACN), heptane-1 sulfonic acid sodium salt (C₇H₁₅NaO₃S), hydrochloric acid (HCl), methanol (MeOH), potassium dihydrogen phosphate (KH2PO4), sodium hydroxide (NaOH), the acetic acid anhydrous (CH3COOH) and phosphoric acid (H3PO4) were purchased from Merck (Darmstadt, Germany) and JT Baker (Center Valley, USA). Deionized and distilled water was used from PURELAB® Ultra Elga DV35 (High Wycombe, United Kingdom). Standards for acetylsalicylic acid [2-(acetyloxy) benzoic acid] was supplied by the Shandong Xinhua Pharmaceutical (P.R. China) and salicylic acid [2-Hydroxybenzoic acid] was supplied by the VWR Chemicals (United Kingdom).
Instrumentation
The HCLASS-UHPLC analysis was performed on both the Waters Acquity with column thermostat and the TUV and Waters Acquity PDA detector with column thermostat (Waters corp., Milford, MA, USA). ACQUITY H-Class System (Waters, Milford, USA) we used to consist of a Quaternary Solvent Manager (QSM), a Sample Manager with Flow Through Needle (SM-FTN) design. The system consisted of the data that were collected and evaluated by using Waters Empower 2 software. To separate acetylsalicylic acid and its active metabolite salicylic acid, a UHPLC column of Waters Acquity was used with BEH, C18, 2.1mm x 50mm with 1.7μm particle size, which was purchased from Waters (Torrance, CA, USA). The column temperature was set as 25οC. The injection port temperature was set at 25oC and the injection volume was 5μl. The total analysis time was 2.5 minutes. All the data for acetylsalicylic acid and its active metabolite salicylic acid were monitored at 280nm.
Samples
It was used Enteric Coated Tablets and its placebo were produced by Abdi İbrahim, Research and Development Center Department (Istanbul, Turkey).
Mobile phase
The mobile phase was prepared by Heptane-1-sulfonic acid sodium salt solution in Acetonitrile: Deionized water (15%:85% v/v) mixture. It was filtered through a 0.45μm membrane filter (Millipore, MA, USA) and degassed by sonication. Acetylsalicylic acid eluted at 1.5 minute in these conditions.
Standard preparations
Stage 1 (0.1 N HCl): A stock solution of acetylsalicylic acid (2.7mg mL-1) and salicylic acid (0.081mg mL-1) were prepared by dissolving an appropriate amount in stage 1 (0.1 N HCl). Working solutions containing 0.081mg mL-1 acetylsalicylic acid and 0.00243mg mL-1 salicylic acid were prepared from this stock solution. It was then filtered through a 0.45μm RC filter and transferred to an HPLC vial.
Stage 2 (pH 6.8 phosphate buffer): A stock solution of acetylsalicylic acid (0.081mg mL-1) and salicylic acid (0.00243mg mL-1) were prepared by dissolving an appropriate amount in stage 2 (pH 6.8 Phosphate Buffer). Working solutions containing 0.041mg mL-1 acetylsalicylic acid and 0.00122mg mL-1 salicylic acid were prepared from 0.1 N HCl. It was then filtered through a 0.45μm RC filter and transferred to an HPLC vial.
Sample preparations
Stage 1 (0.1 N HCI): Place 1000ml dissolution medium into each 6 vessel and warm up to 37οC ± 0.5οC. Drop one Tablet into each vessel and start the analysis at 100rpm paddle speed. At the end of 120 minutes taken sample solution from each vessel. It was then filtered through a 0.45μm RC filter and transferred to an HPLC vial. The drug substances in the final concentrations was 0.081mg mL-1 of acetylsalicylic acid in sample.
Stage 2 (pH 6.8 phosphate buffer): Place 1000ml dissolution medium into each 6 vessel and warm up to 37οC ± 0.5οC. Drop Tablets taken at the end of Stage 1 into each vessel and start the analysis at 100rpm paddle speed. At the end of 90 minutes transfer 5 ml of the solution from each vessel into 10ml volumetric flasks. Dissolve and dilute to volume with 0.1 N HCl. It was then filtered through a 0.45μm RC filter and transferred to an HPLC vial. The drug substances in the final concentrations was 0.041mg mL-1 of acetylsalicylic acid in sample.
Placebo solution
Stage 1 (0.1 N HCI): Place 1000ml dissolution medium into a vessel and warm up to 37 οC ± 0.5οC. An amount of 24.3mg placebo was weighed into a 1000mL dissolution vessel and start the analysis at 100rpm paddle speed. Then, the placebo solution was prepared in the same way as the sample solution.
Stage 2 (pH 6.8 phosphate buffer): Place 1000ml dissolution medium into a vessel and warm up to 37οC ± 0.5οC. An amount of 24.3mg placebo was weighed into a 1000mL dissolution vessel and start the analysis at 100rpm paddle speed. Then, the placebo solution was prepared in the same way as the sample solution.
Results and Discussion
Method optimization
The main purpose of the chromatographic method is the determination of Acetylsalicylic acid and active metabolite salicylic acid, along with maximum selectivity, resolution, retention properties, peak shape and convenient analysis time for the novel analytical method of the enteric-coated tablet dosage form by liquid chromatography according to the ICH guideline 2005 [2]. Different chromatographic conditions were utilized to achieve better efficiency of the chromatographic system. The mobile phase composition, column, detection wavelength, and column temperature were optimized by making necessary changes in the chromatographic conditions to obtain a well-established method. Several proportions of solvents and buffers were assessed to find a compatible composition of mobile phases. The development of the method was initiated by using the USP assay method that could separate Acetylsalicylic acid and active metabolite salicylic acid. This method includes parameters such as an isocratic system, mobile phase consisting of Heptane-1-sulfonic acid sodium salt solution in acetonitrile: deionized water (15%:85% v/v) mixture, the column with Phenomenex Gemini C18, 250mm x 4.6mm, 10μ and heated to 25oC at 280nm wavelength.
Development of the method
Acetylsalicylic acid and active metabolite salicylic acid show the same absorbance with UV spectrophotometric method. Therefore, it was decided to use and develop a more selective HPLC method to measure the amount of acetylsalicylic acid dissolved in enteric-coated tablets containing acetylsalicylic acid. First, dissolution analysis was performed using the assay method conditions in the USP monograph. Due to the conversion of acetylsalicylic acid into active metabolite salicylic acid after 12 minutes of analysis, column size was changed and Phenomenex Gemini, C18, 110A, 4.6 x 150mm, 5μm column was used instead of Phenomenex Gemini C18, 110A, 4.6 x 250mm, 5μm column. In this study, the duration of the analysis was shortened but it was decided to switch to the faster-responsive H-Class system to prevent the degradation of acetylsalicylic acid. As a result of the trial with column Waters, BEH, C18, 2.1 x 50mm with 1.7μm) analysis time decreased to 2.5 minutes and the conversion of acetylsalicylic acid into salicylic acid was prevented. However, the peak separation obtained from the standard injection which was prepared at 0.1N HCl and given to the system for stage 1 was convenient but the peaks obtained from the standard injection which were prepared at pH 6.8 phosphate buffer and given to the system for stage 1 were not convenient. For this reason, organic solvents ratios used in standard solutions have been changed but successful results have not been obtained in this study. Changes in the flow rate have been made by trying different columns. Despite these trials, appropriate peaks were not obtained. So, standard and sample solution prepared at pH 6.8 phosphate buffer at Stage 2 was diluted in an acid medium (0.1N HCl) and injected into the system. As a result of these studies, appropriate peaks were obtained.
Validation of the method
Specificity
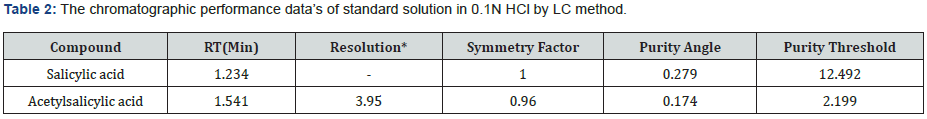
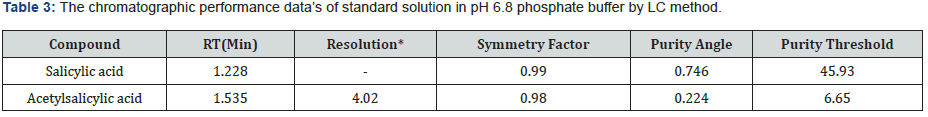
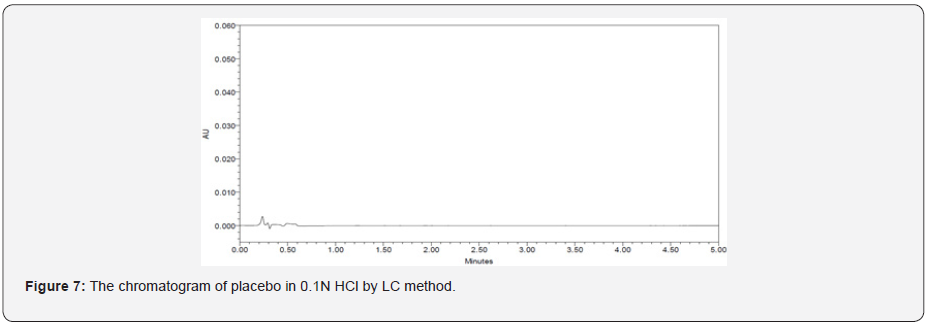
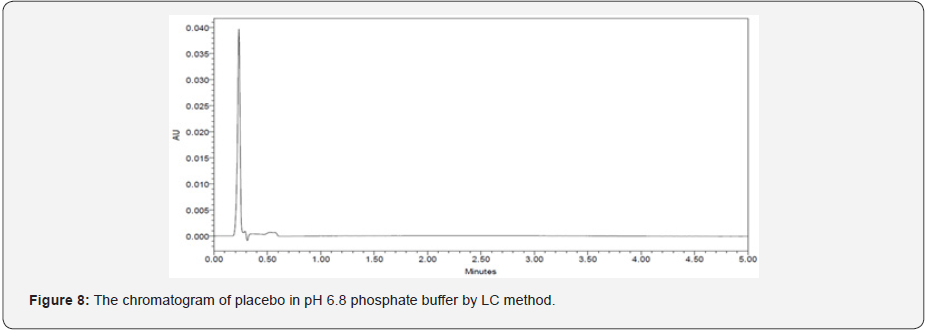
The specificity of a method is its suitability for analysis of a substance in the presence of potential impurities. It can also be used to validate the stability-indicating power of the analytical procedures used. The specificity of the LC method for acetylsalicylic acid has been determined in the presence of salicylic acid impurity (Figure 5 & 6). There was no peak found at the retention time of acetylsalicylic acid and salicylic acid active metabolite in placebo chromatograms proves no interference from placebo (Figure 7 & 8) These results confirmed the specificity of the method (Table 2 & 3).
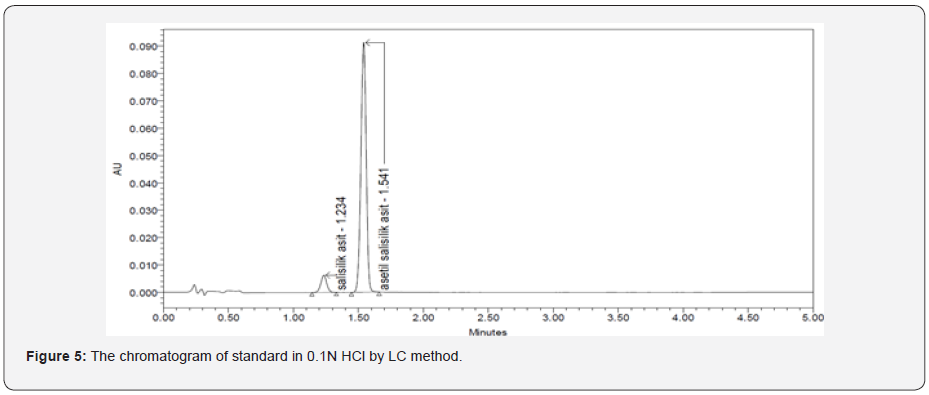
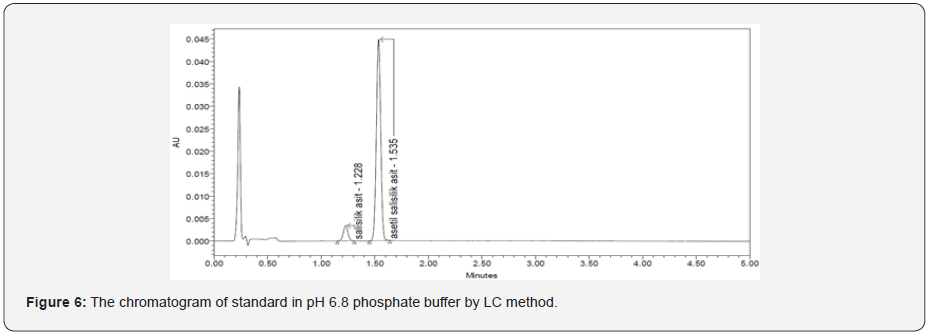
Linearity
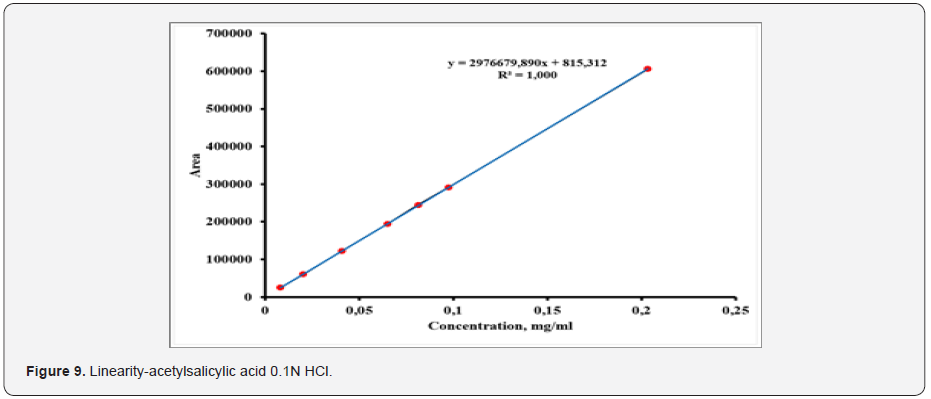
The linearity of the analytical procedure is its ability (within given range) to obtain test results which are directly proportional to the concentration in the sample. The linearity solutions were performed at concentrations levels; 0.0081mg mL-1 - 0.2025mg ml solution of acetylsalicylic acid and 0.0000243mg mL-1 0.00608mg mL-1 solution of salicylic acid corresponding to about 10% to 250%. A linear relationship was established by plotting the peak areas (on Y-axis) against the drug concentration in mg mL-1 (on x-axis). The data obtained in linearity experiments was subject to linear regression analysis. The coefficient of regression (R) was found to be 0.999 for acetylsalicylic acid and salicylic acid. The linearity of the method is proven (Figure 9 & 10).

Precision
The precision of the method verified by system precision, repeatability and by intermediate precision. System precision was checked by Waters Acquity H-Class system with UV detector, Milford, USA) injecting six preparations of standard solution. Repeatability was checked by (Waters Acquity H-Class system with UV detector, Milford, USA) injecting six individual preparations of the sample solution. The intermediate precision of the method was also evaluated using different analyst and different instrument (Waters Acquity H-Class system with UV detector, Milford, USA), and performing the analysis on different days. % RSD of the area for each result was calculated for both precision as well as intermediate precision and was found within 5%. System precision (RSD: 0.06% - 0.12%), repeatability (RSD:1.69%) intermediate precision (RSD: 0.94%).
Accuracy
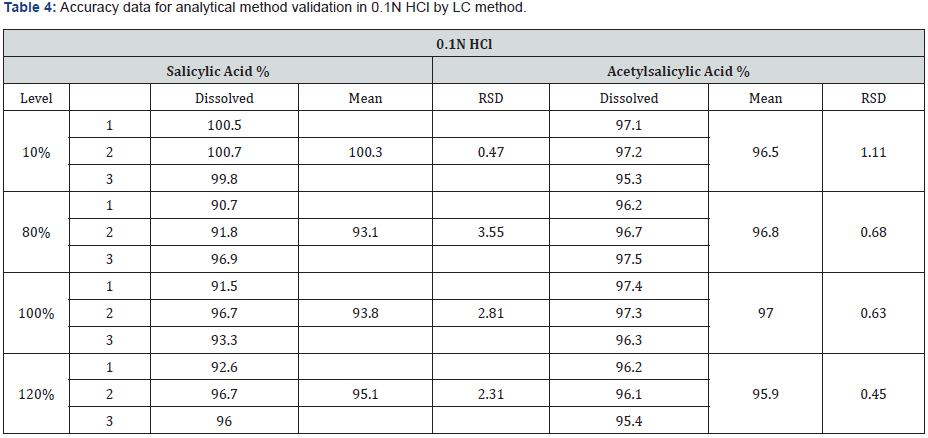
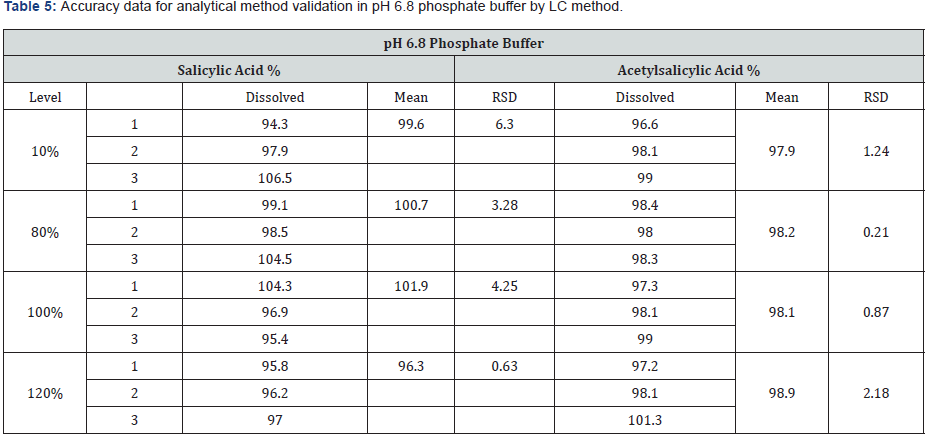
The accuracy was determined by preparation of three replicates of spiked sample solutions for each concentration level. The accuracy of the method was demonstrated. Recoveries for each level were found within the acceptance criteria. (95.3- 97.5%) for stage 1 (0.1 N HCl) and (96.6-101.3%) for stage 2 (pH 6.8 phosphate buffer) The mean recovery of acetylsalicylic acid enteric coated tablet should not be less than 95% and not more than 105%. The results from recovery data are depicted in the (Table 4 & 5).
Robustness
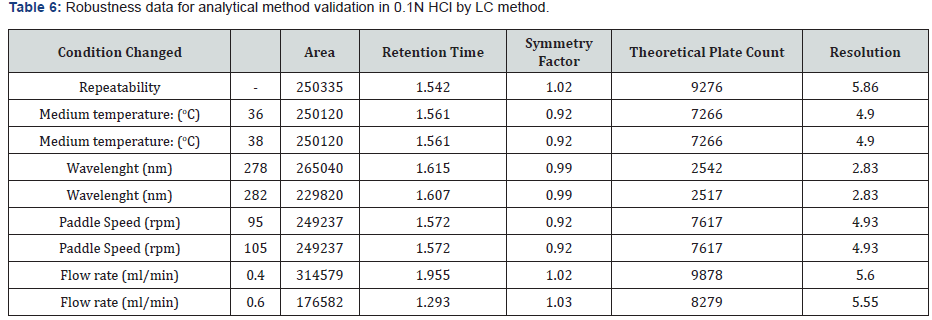
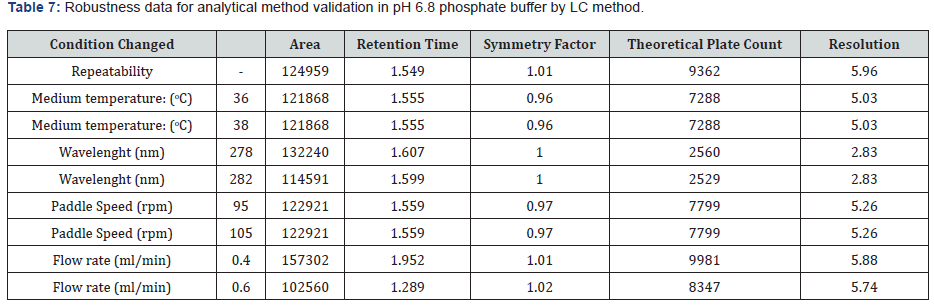
To determine the robustness of the method the experimental conditions were deliberately changed, and the resolution of acetylsalicylic acid and salicylic acid were evaluated. To study the effect of flow rate on the resolution it was changed to 0.4 and 0.6- mL min-¹. The effect of paddle speed was studied at 95rpm and 105rpm. The effect of temperature of dissolution medium was studied at 36οC and 38οC. The effect of wavelength was studied at 278nm and 282nm. Under the different chromatographic conditions, the robustness of the method was proved. The results from the robustness study are depicted in (Table 6 & 7).
Stability in solution
Standard and sample solutions were used for the stability study. No significant changes in the amounts of the acetylsalicylic acid and its active metabolite salicylic acid were observed during solution stability experiments when performed using the dissolution method. The standard and sample solution were found to be stable for 6 hours during the determination of acetylsalicylic acid.
Conclusion
It was developed a more specific method by applying liquid chromatography (LC) to determine both acetylsalicylic acid and its active metabolite salicylic acid. The proposed analytical method has been proved to be simple, specific, precise, linear, robust, rugged, and accurate which fulfill all the parameters of the validation. All calculated statistical values, which were obtained throughout the development and validation of the UPLC method, were within acceptable limits. The method was successfully validated according to the current ICH Guidelines. The method is stability-indicating and can be used for routine analysis of production samples and to check the stability of acetylsalicylic acid enteric coated forms.
To Know more about Pharmacy & Pharmaceutical Sciences
Click here: https://juniperpublishers.com/index.php




This is the post that I was looking for and thank you for sharing.
ReplyDelete