Anesthesia & Intensive Care Medicine - Juniper Publishers
Abstract
Objectives: The aim of this study is describe and analyse bacterial and/or fungal infections in critically ill patients with coronavirus diseases 2019 (COVID-19) pneumonia during the first pandemic period and compared them with historic cohort of influenza pneumonia patients. Design: Multicentre, observational, and retrospective. Setting: Three ICUs of Spain Patients: Critically ill patients with COVID-19 and influenza pneumonia which required ICU admission were recruited from October 2018 to April 30th, 2020. We described population, distribution of infections/microorganisms and risk factors related. Main variables of interest: Exposure: COVID-19 infection. Outcome: Superinfection, Overall mortality 90 days Measurements and Main Results: 58 critical care patients with COVID-19 and a further 48 patients with influenza pneumonia were included. COVID-19 patients had significantly less respiratory co-infection (3 vs 29; p˂0.001). Superinfections were not significantly higher in patients with COVID-19 (46 vs 29%; p=0.06). Gram negative bacteria were significantly higher than in influenza pneumonia patients (48 vs 27; p=0.02) and Pseudomonas aeruginosa (27%), and lower respiratory infections (42 vs 25; p=0.07) were the most frequent aetiology and source of superinfection in COVID-19 patients. In adjusted analysis, days of invasive mechanical ventilation (OR 1.08; CI 1.01-1.16; p=0.04) and SOFA score at admission (OR 1.48; CI 1.13-1.95; p=0.04) were statistically associated with superinfection. Superinfection (HR 4.76; CI 1.63-13.91; p=0.004) was related in crude analysis with overall mortality at 90 days. Conclusion: The incidence of coinfections is low in critically ill patients with COVID-19 pneumonia and significantly less frequent than in patients with influenza pneumonia. Empirical treatment at admission should be closely revaluated. Incidence of superinfection is high, if is suspected, empirical antipseudomonal treatment should be considered specially in lower respiratory infections, considering local flora. Severity of illness and supportive treatments seems to be the clearer factors related to superinfections.
Keywords: Coronavirus infection, Pneumonia, Infection, Coinfection, Superinfection, Critical care
Introduction
Information about bacterial and fungal infections in critical patients with Coronavirus disease 2019 (COVID-19) is increasing after one year of the pandemic outbreak. Some explanations of this fact are the most immediate priorities for hospitals providing acute medical care (intensivist have habilitated up to 300% more critical patients’ beds in hospitals), keeping critically ill patients alive, protecting staff, and optimizing care of critically ill non-COVID patients in this context [1-3].
Coinfection in patients with COVID-19 seems to be low according to published data, but complications with superinfections in these critically ill patients shows important variability in different studies [4]. While some reports show low incidence of hospital acquired pneumonia of 11%, others show an increase of incidence of more than 50% of lower respiratory infections or high incidence of blood stream infections in these patients [5,6].
The aim of this study is to describe bacterial and/or fungal coinfections and superinfections in critically ill patients with COVID-19 pneumonia, compare them with critical care patients with influenza pneumonia and evaluate the risk factors associated. This knowledge may be useful to improve management of these patients, optimize antibiotics policy and to adapt initial protocols.
Patients and Methods
This is an observational, retrospective, cohort study carried out at 3 hospitals in the south of Spain. We evaluated all adult patients with viral pneumonia due to SARS-VoV-2 and influenza who required ICU admission from October 2018 to April 30th, 2020. Eligibility criteria included were age ≥18 years old, confirmed laboratory test of SARS-COV-2 or influenza (A or B) infection, and compatible chest X ray or computed tomography infiltrates. Patients with limited therapeutic effort at admission and ICU hospitalization duration lasting ˂48 hours were excluded.
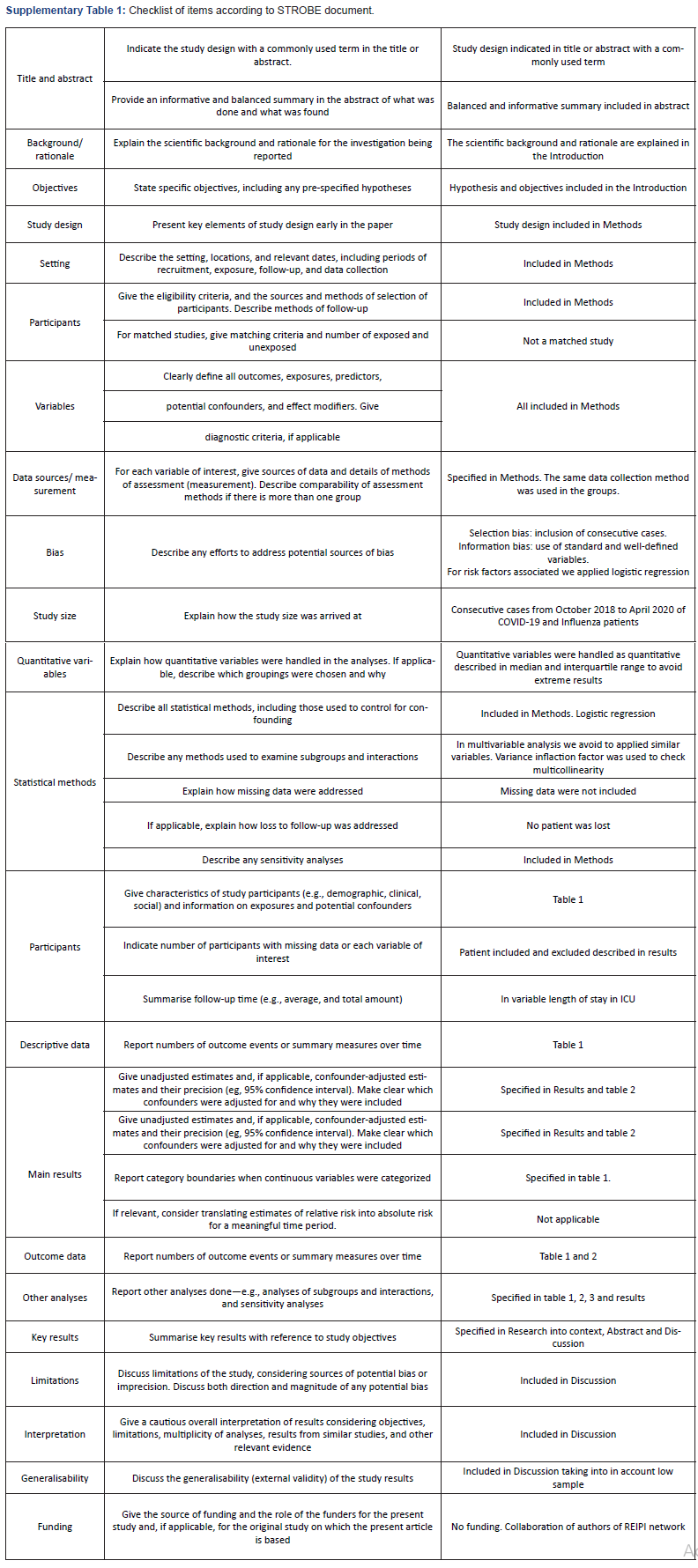
The study was approved by the local Ethics Committee of the Reina Sofía University Hospital of Córdoba (Code 4706), which were exempted from the need to seek written informed consent due to the observational and retrospective nature of the study. STROBE recommendations for observational studies were followed and checked (Supplementary Table 1) [7]
Clinical data was recorded in a standardized protocol and anonymized in a database. Variables evaluated were: (i) demographics, (ii) previous conditions, (iii) clinical characteristics, (iv) severity of illness (APACHE II and SOFA score) (v) analytic and microbiological studies, (vii) pharmacologic and support therapy, (viii) and outcome variables (microbiologically confirmed coinfection and superinfection during ICU stay, type of infection, microbiology characteristics, overall mortality at 30 and 90 days and died with active infection). Corticosteroids when administered were given at maximum doses of 1mg/kg/day of methylprednisolone. All patients admitted during this period were followed until ICU discharge and overall mortality 90 day was assessed by phone call in discharge patients.
To standardize the classification of an infection as present on admission (co-infection) or a healthcare-associated infection (superinfection), we used the CDC/NHSN criteria. Superinfection is defined as localized or systemic condition resulting from an adverse reaction to the presence of an infectious agent(s) or its toxin(s) that was not present on or after the 3rd hospital day (day of hospital admission is day 1) [8]. Diagnosis of lower respiratory infection was based on two of the next criteria: temperature up to 38.5 ˚C or leucocyte account (≥12.000 or ˂4.000 cells per μL), and purulent secretions. Episodes need microbiological confirmation with isolation in the endotracheal aspirate. Ventilator associated pneumonia was defined with the above criteria and the presence of new or progressive infiltrates on radiological explorations after 48 hours of mechanical ventilation [6]. ICU bloodstream infection for typical skin contaminants (e.g. coagulase-negative Staphylococcus) were included only if ≥2 blood cultures showed the same phenotype on separate occasions, or 1≥ blood cultures for clinical sepsis and no other infectious process; catheter related bloodstream infections were documented by quantitative tip culture [9]. All episodes and criteria were critically reviewed by two investigators (A.M and J.R.G).
SARS-COV-2 testing was performed at Microbiology Reference Laboratory (Reina Sofía University Hospital/IMIBIC). VIASURE SARS-CoV-2 Real Time PCR Detection Kit (CerTest Biotec), a multiplex real-time PCR detecting ORF1ab and N genes, was used for diagnosis. Influenza A/B virus were also investigated using GeneXpert® (Cepheid) or the BioFire® FilmArray® Respiratory 2 plus Panel (Biomerieux).
Continuous variables were compared using the Mann- Whitney U or T Test of Student Test and categorical variables were compared using the Chi Square Test or Fisher’s Exact Test as appropriate. Ventilator associated pneumonia and blood stream infections incidence rate were calculated as the number of events per 1000 patient-days at risk; the 95% confidence interval (CI) for the incidence rate estimate was obtained using mid-P exact test. In patients with COVID-19, multivariate analyses were performed using logistic regression for study variables related with superinfection and Cox regression for overall mortality 90 days. In logistic regression for variables related to superinfection, each multivariate analysis included one variable for every ten events; they were selected those with p˂0.1 (model 1) or those with clinical interest (model 2 and 3). Due to prolonged length of ICU stays of COVID-19 patients we decide overall 90 days mortality over overall 30 mortality. In Cox regression analysis for overall mortality 90 days, superinfection variable was included as time-dependent; patients have an additional transient state before superinfection event, not taking timing, this time, into account would cause time-depending bias [10].
Results
Description of patients with COVID19 and Influenza pneumonia
During the study period 116 patients were evaluated. 62 patients with highly suspected COVID-19 pneumonia were identified; 58 met inclusion criteria, 3 patients with COVID-19 disease like symptoms were not confirmed in laboratory RT-PCR of SARS-COV-2 and 1 patient with limited therapeutic effort at admission was excluded. There were 48 influenza pneumonia patients evaluated and all of them were legible without exclusion criteria.
Clinical characteristics of COVID-19 and influenza pneumonia cohorts are described in Table 1. Critically ill patients with COVID-19 pneumonia were median age 64 years old. Only 10% (6) of patients did not have comorbidities while the most frequent were hypertension and obesity with 56% (33) and 37% (22) respectively. At admission, laboratory results showed the median of D Dimer of 1096 ng/ml, median of 347 cell/ μL of CD4 and 47 pg/mL of IL6.
Patients with COVID-19 in comparison with those with influenza pneumonia were significantly older (64 vs 55; p=0.004), with higher incidence of hypertension (56 vs 37; p=0.04), more days from onset of symptoms to ICU admission (9 vs 4; p˂0.001), treated with corticosteroids (43 vs 16%; p=0.002), but have less APACHE II score (12 vs 19; p˂0.001).
Co-infection and superinfections
Patients with COVID-19 pneumonia, in comparison, had significantly less frequent respiratory co-infection (3 vs 29; p˂0.001) (Table 2). 96% of patients with COVID-19 were treated with antibiotics at admission. There were 3 cases of Coagulase Negative Staphylococcus in COVID-19 patients at ICU admission.
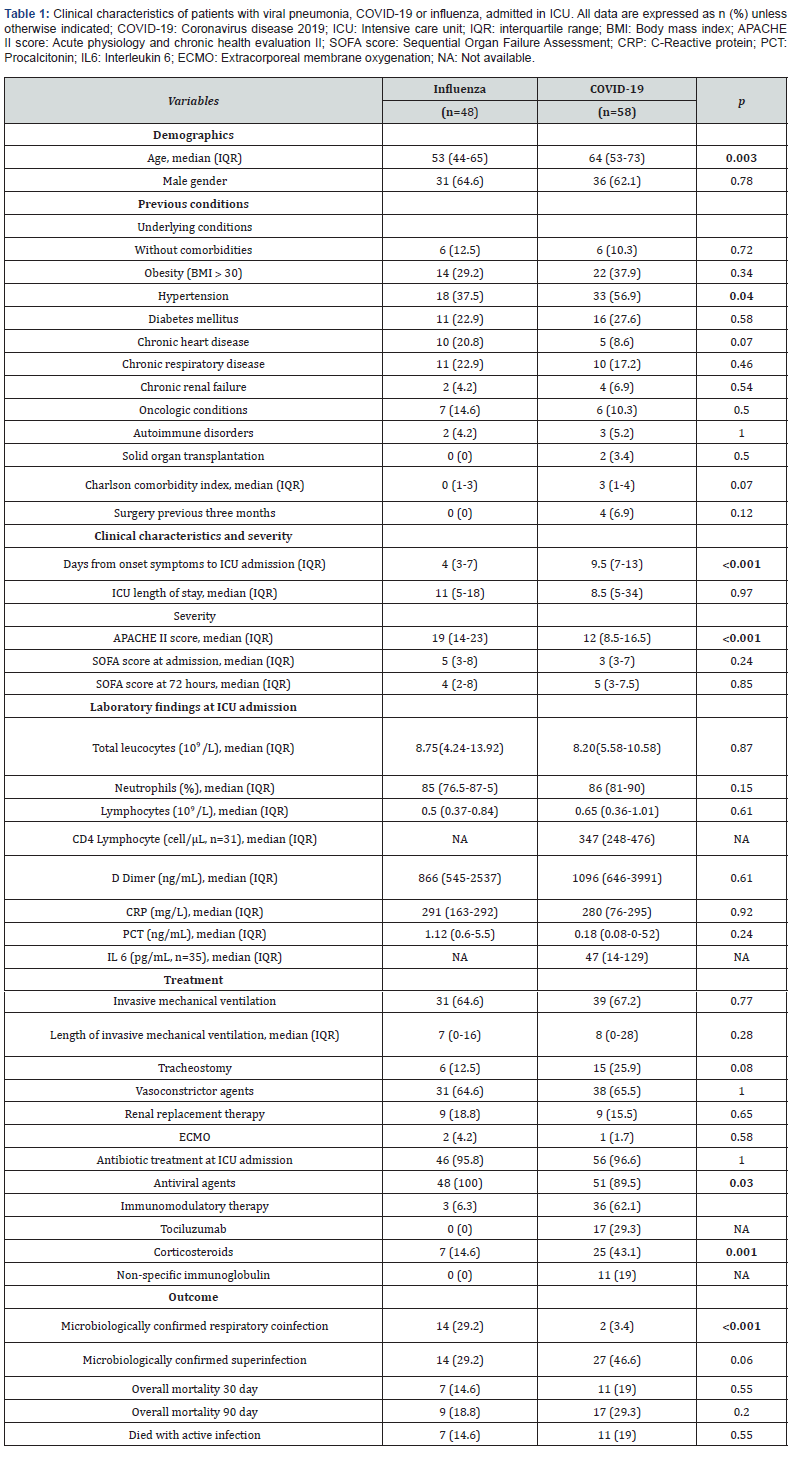
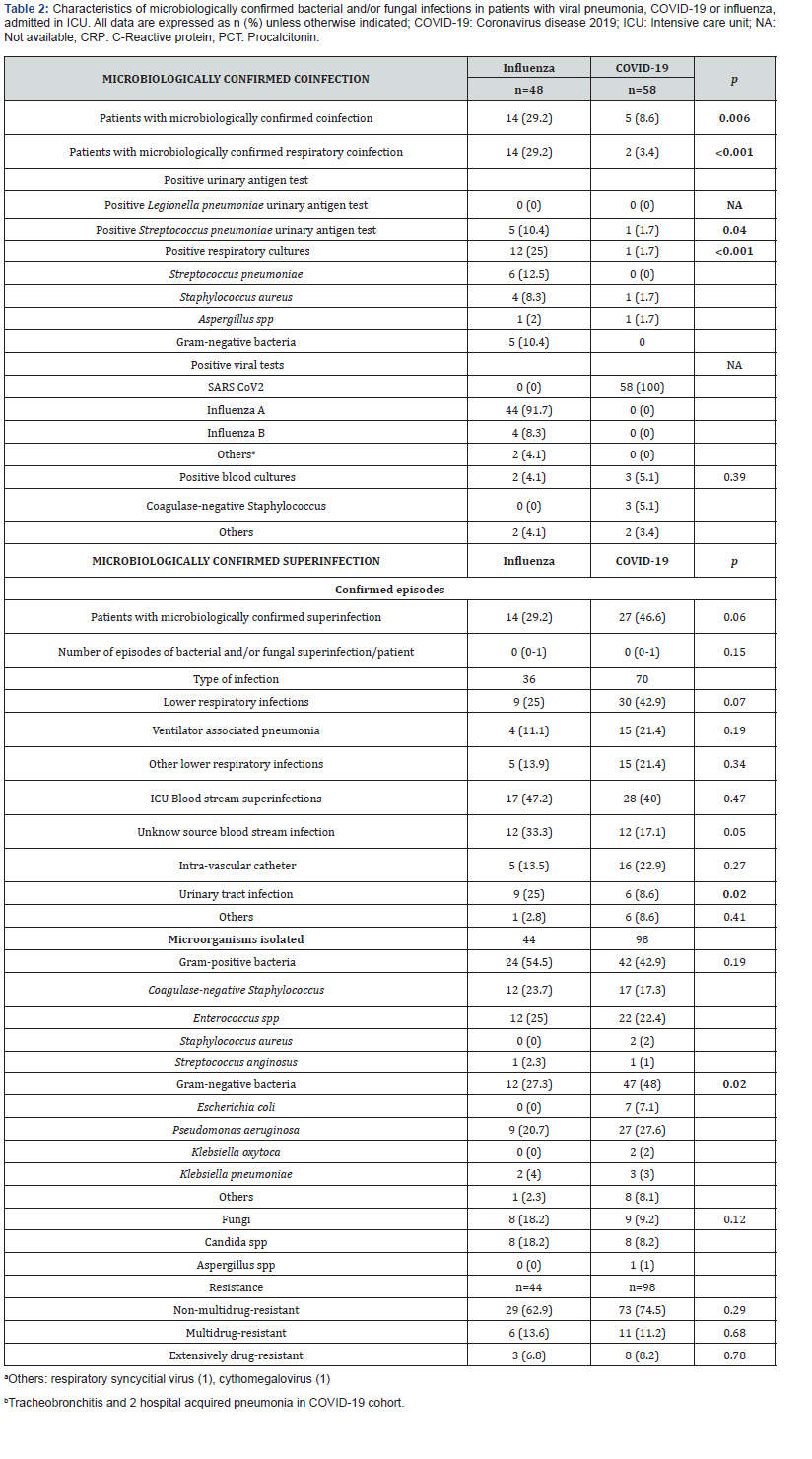
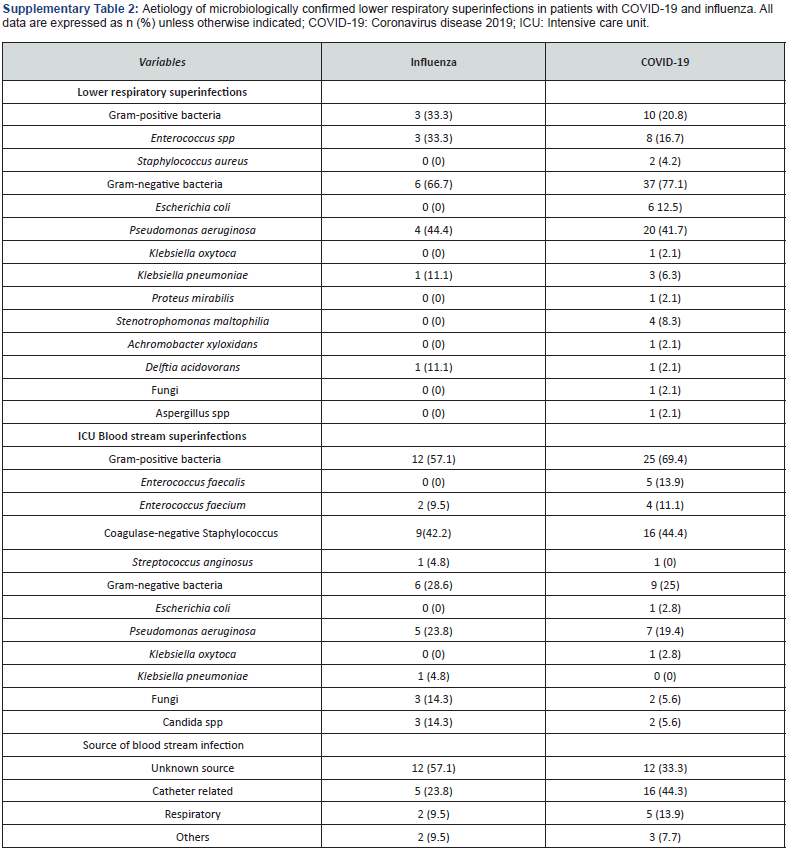
Superinfections were not significantly higher in patients with COVID-19 (46 vs 29%; p=0.06). The most frequent microorganisms isolated in this cohort was Pseudomonas aeruginosa (27%), and Gram-negative bacteria were significantly higher than in influenza pneumonia patients (48 vs 27; p=0.02) (Table 2). Lower respiratory infections were the most frequent superinfections in COVID-19 patients, and they were not significantly higher than influenza pneumonia patients (42 vs 25; p=0.07); gram negative (77%) and Pseudomonas aeruginosa (41%) were the most frequent microorganisms related in this infection. (Supplementary Table 2) Incidence rate of ventilator associated pneumonia was not significantly higher in this group with 13.2 (CI95% 7.24-21.8) per 1000-days-ventilated COVID-19 patients in comparison with 7.1 (CI 1.9-18.3) per 1000-days-ventilated patients in influenza pneumonia cohort (p=0.28). ICU blood stream superinfections were similar in both cohorts (40 vs 47%; p=0.47) with and incidence rate (including secondary bacteraemia) of 25.8 (CI95% 18.1-35.8) in COVID-19 patients and 24,3 (CI95% 15.1-37.2) per 1000-days-at risk in influenza pneumonia cohort (p=0.84). Grampositive bacteria and Coagulase-negative Staphylococcus were in both cohorts the most frequent bacteria (Supplementary Table 2). The rate of blood stream candidemia was 5 and 14%.
Variables related with bacterial and/or fungal superinfection in critical care patients with COVID-19
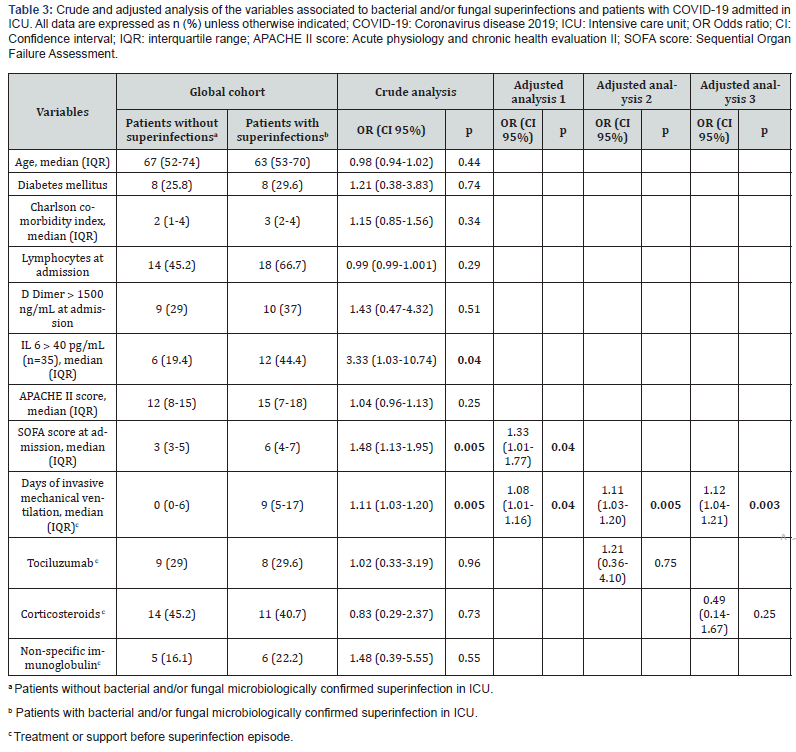
The crude and adjusted analysis of the association between selected variables and superinfection in COVID-19 patients is shown in Table 3. In the crude analysis, variables statically significant related to superinfection were the severity of the illness at admission, SOFA score (Odds Ratio (OR) 1.48; CI 1.13- 1.95; p=0.005), interleukin 6 (OR 3.33; CI 1.03-10.74; p=0.04) and days of invasive mechanical ventilation (OR 1.11; CI 1.03- 1.20; p=0.005). In adjusted analysis, days of invasive mechanical ventilation (OR 1.08; CI 1.01-1.16; p=0.04) and SOFA score at admission (OR 1.48; CI 1.13-1.95; p=0.04) were statistically associated with superinfection. Treatments with tociluzumab (OR 1.21; CI 0.36-4.10; p=0.75) or corticosteroids (OR 0.49; CI 0.14- 1.67; p=0.25) failed to show any relation in the crude and adjusted analysis.
Prognosis in COVID19 patients: Impact of superinfection
Patients with COVID-19 showed higher overall mortality 90 day but there were not statistical differences in comparison with patients with influenza infections (29 vs 18%; p=0.20). Eleven patients died in treatment with active infection in COVID-19 cohort. Survival analysis of 90-day overall mortality did not show differences between COVID-19 and influenza pneumonia (p=0.20). (Supplementary Figure 1)
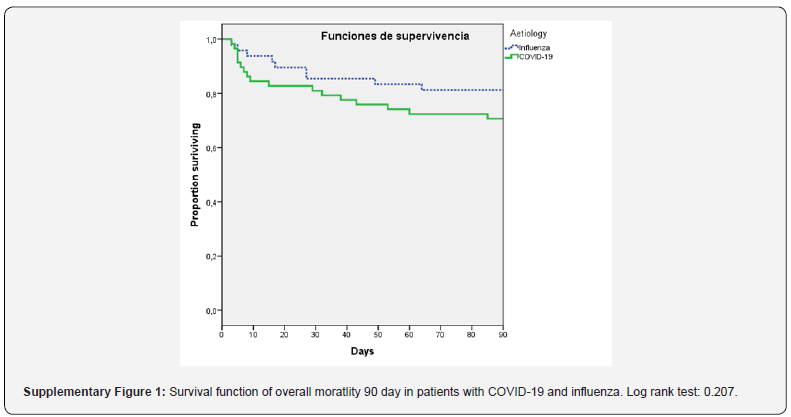
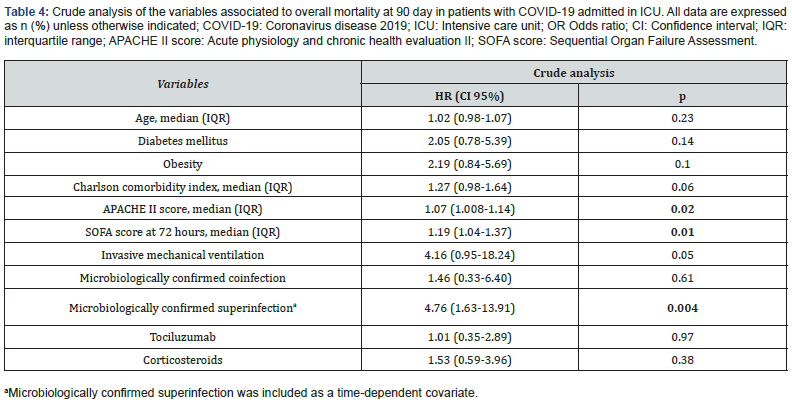
Table 4 shows that variables that were related in crude analysis with overall mortality at 90 days were severity of illness, APACHE II score (HR 1.07; CI 1.008-1.14; p=0.02), SOFA score at 72 hours (HR 1.19; CI 1.04-1,37; p=0.01) and superinfection (HR 4.76; CI 1.63-13.91; p=0.004) when was explored as timedependent variable.
Discussion
We found that in our cohort of critically ill patients with COVID19 pneumonia, patients experienced low coinfection rate (3%) and high incidence of superinfections (46%). Gram negative infections were significantly higher in comparison with those with influenza pneumonia and lower respiratory infections and Pseudomonas aeruginosa were the most frequent source and microorganism related.
Microbiologically confirmed respiratory co-infection of COVID-19 patients of our cohort was significantly lower (3%) than patients with influenza pneumonia. Similar findings are noted in other studies, so we could suggest that coinfection is unlikely to be common in patients with COVID-19 upon admission to the ICU [11,12]. On the other hand, we are aware that coinfection could be influenced by seasonal factors and it is unknown if these results could change during other periods. Considering the low coinfection rate, empiric antibiotic treatment should be closely revaluated once bacterial and/or fungal bacterial infections has been ruled out to reduce resistant emergence. We found 3 cases at admission of bloodstream infections by Coagulase-negative Staphylococcus; in the context of fulfil bloodstream infection criteria and clinical systemic inflammatory response they were treated. We could not rule out that those results as contaminations.
The published incidence of superinfections in critical care patients with COVID-19 is highly variable. While some reports show low incidence of lower respiratory infections less than 30%, other studies point to SARS-CoV-2 as an independent risk factor and to increase its´ incidence (˃50%) [5,6,13,14]. In our study, the incidence of lower respiratory infection is elevated (42% of lower respiratory infection and 21% of ventilator associated pneumonia) and higher than those patient with influenza pneumonia. Even though we found lower incidence rate of ICU bloodstream infection than other reports like Giacobbe et al. [15] study (47 vs 28 episodes per 1000 patients-days at risk in our COVID-cohort), and was not significantly higher than influenza patients, still remains high with up to 20% incidence of catheter related blood stream infections [15].
Some reasons that could explain this high variability between studies may be: different pressure of ICU admissions and its´ impact in quality care, publication bias (tend to report good results), different severity of illness and patients characteristics, the effect of mortalities differences in superinfection incidence (competitive risk bias) and exclusion in some studies of patients that are still admitted to hospital or ICU at the end of the studies follow up, which could corresponded with long term patients with high rate of complications [12-14,16].
Critical COVID-19 patients are exposed to important risk factors for superinfection that can explain the high rate established like advanced age, underlying systemic diseases, acute respiratory distress syndrome, mechanical ventilation, catheters and prolonged hospital and ICU stays [17,18]. In our case we found that the severity of presentation (SOFA score at admission) interleukin 6 and supportive treatment (days of invasive mechanical ventilation) showed to be more important factors associated with superinfections in that of critical care patients with COVID-19. Even though we were unable to assess if immunomodulatory treatment was related, the study is not powdered enough to test this hypothesis; published data shows controversial findings related corticosteroids and tociluzumab: the balance of the result of theatrically reduction of supports with immunomodulatory treatment (e.g. mechanical ventilation and prognosis) and the increase of the exposure to superinfections is still unknown [9]. Some studies point to SARS-COV-2 as an independent risk factor for exposure to lower respiratory and blood stream infections. Suggested explanations for this hypothesis are endothelial dysfunction, widespread thrombosis, histologic findings of acute fibrinous and organizing pneumonia and gut biota disruption [6,9].
In some papers increasing Pseudomonas aeruginosa superinfections in COVID-19 patients were noted [6,19]. In our case, Pseudomonas aeruginosa was too the main pathogen isolated (27%). Future studies will assess if COVID-19 clinical evolution and its´ histopathology lung damage/ADRS may predispose to these kinds of infections; despite this, we found that gram negative infections were significantly higher in COVID-19 patients in comparison with influenza pneumonia, local flora may play an important role in this issue.
In our case we can show that superinfection was independently associated with overall mortality at 90 days in crude analysis, but future studies will clear the complex relationship between severity of illness, superinfection, treatments, and mortality.
Our study has obvious limitations. Its´ observational design is exposed to certain biases by its nature, even though we tried to minimize them by applying strict definitions and following STROBE recommendations for observational studies. We are aware of the important limitation of sample size; that is important in the precision of our estimate, so it must be taken with caution. Epidemiology may be influenced by local flora. This cohort of critical care patients with COVID-19 were admitted to ICU before RECOVERY trial results, so more than one half of patients did not receive corticosteroids, it can impact in the future patients and superinfections events [20]. But considering the context of the high variable data reports, these results in this critical care cohort specifically focused on bacterial and/or fungal infection in COVID-19 patients may be useful discussing issues such as the co-infection and superinfection, it´s impact and epidemiology and treatment approach.
Conclusion
The incidence of coinfections is low in critically ill patients with COVID-19 pneumonia and significantly less frequent than in patients with influenza pneumonia. Empirical treatment at admission should be closely revaluated. Incidence of superinfection is high, if is suspected, empirical antipseudomonal treatment should be considered specially in lower respiratory infections, considering local flora. Severity of illness and supportive treatments seems to be the clearer factors related to superinfections.
To Know more about Anesthesia & Intensive Care Medicine
Click here: https://juniperpublishers.com/index.php




No comments:
Post a Comment