Drug Designing & Development - Juniper Publishers
Opinion
The pandemic of innovative coronavirus disease (COVID-19) is an unparalleled public health danger. The disease was discovered in late December 2019 in Wuhan, Hubei Province, China, with an exceptionally high rate of spread. The virus that causes COVID-19, identified as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has infected over 118 million people and caused 2.5 million deaths in 216 countries, with the numbers continuing to rise. There are currently no officially licensed vaccines or antiviral drugs for the treatment or prevention of COVID-19. Numerous therapeutic techniques have been tried since its discovery, such as the use of repurposing drugs including broad-spectrum antivirals, immunomodulators, plasma therapies, protease inhibitors, and nucleoside analogs, among others. However, these methods have not demonstrated substantial clinical benefits and are only used to relieve symptoms. Nanotechnology has evolved in recent decades, allowing us to build new nanomaterials and progress in the application of new technical tools.
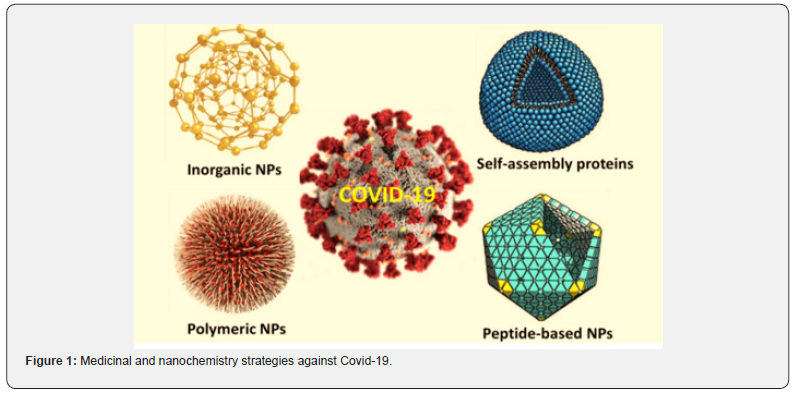
Nanotechnology-based clinical solutions are a potential way to get around the limitations of COVID-19 prevention, diagnosis, and treatment [1]. Nanomaterials with antiviral activities may be used in personal protective equipment and disinfection procedures to avoid SARS-CoV-2 infection [1], as well as nanomaterials-based vaccines or immunomodulators, to control this disease [1,2]. Nanomaterials can also be used in diagnosis to develop simple, fast, and low-cost detection methods for SARS-CoV-2. For care, nanosystems are used to deliver antiviral and biomolecules to the pulmonary system in a controlled manner, for example, to prevent viral replication or prevent the growth of viral particles [1]. Nanotechnology has the ability to have major benefits in the treatment of emergency viral diseases like COVID-19 [3]. Nanoscale structures, as opposed to macro and micro components, have different physicochemical and biological properties, as per the Food and Drug Administration (FDA). Drug encapsulation in nanocarriers, for example, allows for more precise monitoring of drug release in target sites, increased biocompatibility, and decreased toxicity in healthy tissues [4]. Nanomaterials also prevent infectious infection from the air and interaction with contaminated objects, which could be particularly useful in the sterilization of protective equipment and in a hospital setting. Nanomaterials also have optical and electrical properties that have been studied extensively in the development of diagnostic tools, such as point-of-care (POC) biosensors. Nanoparticles, which allow us to identify the study at low concentrations, may boost the sensitivity of detection. These techniques aid in the rapid diagnosis and exclusion of SARS-CoV-2 infected individuals, as well as the treatment of those infected. Nanomaterials’ interactions with biological interfaces are the framework for their future (bio) medical applications [5]. By allowing new and improved ways of prevention, diagnosis, and treatment, nanotechnology opens up new possibilities in the battle against COVID-19 based on the properties and advantages of nanostructured systems (Figure 1).

Gold nanoparticles (AuNPs) are one of the most appealing nanomaterials because they have optoelectronic properties that can be investigated by a number of biosensors, including spectrofluorimetric, electrochemical, and plasmonic detection systems [6]. AuNPs have been commonly used to mark the target molecule in lateral flow assays (LFAs), resulting in a color shift in the test zone that can be read by the naked eye for qualitative results or analyzed by a smartphone that can assess the concentration of the target from a recorded picture [7].
LFAs biosensors have recently been used to diagnose COVID-19 and are now being distributed to healthcare systems to allow for highly centralized testing [9]. Other colorimetric monitoring systems, in addition to LFAs, investigate the redshift in the LSPR band of AuNPs when they collate. The most main characteristic strategy is to functionalize these nanomaterials with biomolecules, which causes the AuNPs to aggregate and cause a color change in the solution when they bind to the target molecules (Figure 2). Importantly, because it is based on the optical properties of AuNPs, this colorimetric assay can significantly contribute to COVID-19 management by providing a quick and simple diagnosis.
Nanomedicine is a valuable resource in the fight against innovative coronaviruses, but its application in clinical practice still faces significant challenges, most notably in vivo behavior, nanocarrier toxicity, and industrial scale production. Other critical issues include a lack of understanding of the specific characteristics and aspects of disease physiopathology, the processes involved in the nano-biointerface, as well as cytocompatibility, safety, and regulatory issues. COVID-19-specific features and the physicochemical properties of nanosystems can be investigated and used to design customized nanostructures for specific therapeutic purposes, with the goal of neutralizing the current threat to global public health and developing more sustainable nanotechnology-based approaches. Although these factors have not yet been thoroughly investigated, they are critical for the safe and effective application of nanotechnologies to combat SARSCoV- 2 infection.
To Know more about Drug Designing & Development
Click here: https://juniperpublishers.com/index.php




No comments:
Post a Comment