Recent Advances in Petrochemical Science - Juniper Publishers
Abstract
Biodiesel is a promising candidate fuel for traditional petroleum diesel fuel (derived from crude oil). The efficient production of biodiesel depends on various parameters which are involved in its preparation from its constituents, i.e., oil, methanol, catalyst and the total energy input (reaction time and temperature). This review highlights some recent developments in nanoparticles-based heterogeneous catalysts in biodiesel production. This involves the use of CaO-based catalysts derived from various sources. The operating conditions as well as the biodiesel yield are concisely highlighted.
Keywords: Biodiesel Renewable energy Heterogeneous catalysis Nanostructures
Background
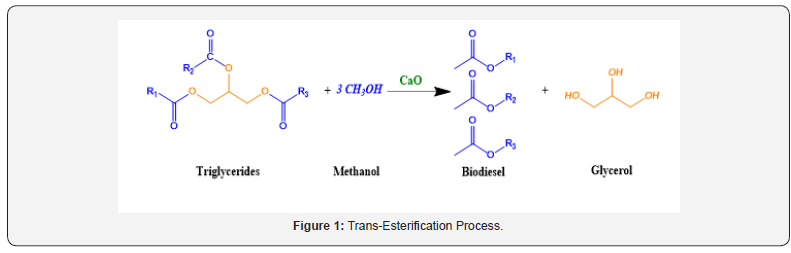
Biodiesel is a promising alternative fuel for diesel vehicles and machines. Biodiesel is a renewable fuel which is characterized by low emission of the harmful nitrogen oxides and sulfur oxides gases, i.e., NOx and SOx compared to the traditional petroleum diesel. Thus, the use of biodiesel supports cleaner environment when it comes out the exhaust pipes and thus helps reduce global warming. Biodiesel production is growing world-wide due to the increasing desire for energy, and interest in environmentally friendly fuel production. It is gradually replacing petroleum diesel in several city buses, and some governmental fleets in the united states and elsewhere. It is basically the methyl ester of fatty acids which is prepared via the reaction between oil (triglyceride-fatty acid source) and methanol in such a way that methanol replaces the glycerol in what-is-called a trans-esterification process according to the following equation: Several parameters control the speed of the forward reaction towards the production of biodiesel. These include the amount of methanol, reaction temperature and time in addition to the presence of catalyst. Figure 1 above shows that a stoichiometric ratio of oil to methanol should be at least 1 to 3. Thus, a considerably sufficient amount of methanol should be added to the reaction mixture to ensure the high conversion of oil (triglyceride) to biodiesel. Also, each gallon of produced biodiesel requires at least one gallon of oil feedstock.
Keywords: Biodiesel Renewable energy Heterogeneous catalysis Nanostructures
Catalytic Aspect of Biodiesel Production
The kinetics of this un-catalyzed reaction (Figure 1) is slow. That is a catalyst should be used to push the forward reaction towards biodiesel production in a measurable rate. In this context, several catalysts were suggested including homogeneous catalysts (e.g., KOH and H2SO4) and heterogeneous catalysts (mainly basic oxides, e.g., CaO, MgO). The use of homogeneous catalysts furnishes the advantage of short reaction time and high reaction yield of biodiesel could be achieved [1-2]. But a major disadvantage is the corrosion of the reaction container, foaming (soap formation) together with the difficulty of separation of the homogeneous catalyst from the produced biodiesel and the huge amount of wastewater resulting from its washing, leading to a substantial increase in the overall cost of the process [3]. This is where heterogeneous catalysts show their superiority as a promising alternative particularly basic metal oxide (e.g., CaO, MgO, …. etc.). Thus, CaO-based catalysts showed a significant potential performance towards the forward direction of the trans-esterification process. CaO obtained from agricultural and industrial waste residues are introduced as commercial sources of CaO-based catalysts. The use of CaO solid based catalysts safes the environment, on the one hand, and high biodiesel production efficiency, on the other hand [4-5]. Recently the trans-esterification of vegetable oils to biodiesel has been successfullyachieved [6-9] using residues of paper mill industry [10], eggshell [5,11], sea creatures shell [12,13], animal bones [14-16] and plant ashes [17-19]. Recently, sugar beet agro-industrial waste showed superior activity towards biodiesel production via the transesterification reaction of sunflower oil with methanol [20]. The sole role of CaO is the acceleration of the replacement of glycerol by methanol via catalyzing the several elementary steps involved in the trans-esterification process [20].
The use of nanoparticle-based materials has been emerged as efficient catalysts in several chemical as well as electrochemical reactions. This is because of the high surface area associated with the use of materials in nanometer scale dimensions, e.g., nanoparticles, nanorods, nanospheres, nano cubes. Moreover, the electronic as well as the surface properties of materials are significantly differing in this tiny dimensions. For instance, gold nanoparticles based (AuNPs) catalysts showed a superb catalytic enhancement for low temperature oxidation of carbon monoxide as reported by Haruta et.al. [21]. Furthermore, AuNPs showed excellent electrocatalysis for the oxygen reduction reaction in alkaline, acidic and neutral media [22-24]. Using the virtues of nanoscale material, CaO-based catalysts were prepared in thistiny size dimension to catalysis the trans-esterification of oil to biodiesel (Figure 1). Abdelhady et. al. [20] prepared CaO-based nanocatalysts from sugar beet agro-industrial residue by thermal treatment. They showed that the calcination temperature plays a prominent role in determining the particle size, chemical composition as well as the surface area of the prepared catalyst. The optimum conditions for biodiesel production are shown using 1 wt% CaO nanoparticle-based catalyst (calcined at 800oC) after refluxing oil/methanol blend (molar ratio of 0.22) for 1 hour at 75oC. Whereas, Empikul et. al. [25] utilized 10 wt% of CaO-based catalyst (obtained from eggshell waste) for biodiesel production using palm olein oil and a high methanol to oil ratio (18:1) yielding ca. 94% in 2 hours and at 60°C. Similarly, Li. et. al. [11] utilized 6 wt% of CaO obtained from paper mill industry waste for transesterification of peanut oil into biodiesel (94%) under similar reaction conditions. Also, Correia et. al. [26] used crab shell residue as a source of CaO for the transesterification of sunflower oil in the presence of methanol with a biodiesel yiled of ca. 83% after reflux for 4 hours at 60°C. Additionally, Smith et. al. [17] used bovine bone as a source of CaO-based catalyst (with 8 wt% loading level) and a biodiesel yield of about 97% was obtained after 4 hours’ reflux at 65oC.
Concluding Remarks
This review highlights the significant advances in the use of Ca oxide-based catalysts derived from natural sources (e.g., wastes and residues from several agricultural and industrial activities) as promising heterogeneous catalysts for the production of biodiesel employing various oil feedstocks in the presence of methanol at acceptable reaction conditions expressed in reaction time and temperature. The suggested CaO-based heterogeneous catalysts showed superior activity towards biodiesel production compared to homogeneous catalysts (i.e., in solution phase) in terms of reusability, ease of separation, and milder aggressive corrosive effects on the reaction vessel and operating apparatus.
Click here: https://juniperpublishers.com/index.php




No comments:
Post a Comment