Pharmacy & Pharmaceutical Sciences - Juniper Publishers
Abstract
Obesity is a chronic disease associated with serious health problem such as metabolic syndrome, diabetes, hypertension, and cardiovascular disease. Due to the growing trend of returning to nature and the fear of adverse reactions from conventional medicines, people are increasingly resorting to the use of herbal preparations.
Adulterated herbal weight loss products with containing undeclared synthetic drugs are common and responsible for many serious health damages. Because of long-term use and natural origin these preparations give a sense of security. But herbal weight loss formulations also possess undesirable effects and, among other dangers, present a risk connected with deliberate addition of synthetic compounds, deliberate or unintentional replacement of the plant species or simply a risk of mislabeling.
The most undeclared ingredients, which were illegally added include sibutramine, phenolphthalein, bumetanide, and phenytoin in the herbal called products, weight reducing and fat loss supplements. Caffeine, pseudoephedrine, theobromine and amfepramone were also found in the supplements. In the world of Internet ordering, these are the dangers that everyone should be aware of. In this article, we reviewed the safety issues related to adulterated or mislabeled herbal products.
Adulterated synthetic substances were detected in the herbal weight loss products. Health care professionals should make people aware of the risks of taking herbal weight-loss supplements.
Keywords: Adulterations; Limming products; Herbal weight-loss; Detection methods
Abbreviations: 5HT: Serotonin; CE: Capillary Electrophoresis; CV: Cardiovascular; DA: Dopamine; HPLC: High Pressure Liquid Chromatography; GC: Gas Chromatography; NA: Norepinephrine; RCT: Randomised Controlled Trial
Introduction
Obesity, defined as abnormal or excessive fat accumulation and a Body Mass Index (BMI) above 30 kg/m. The prevalence of obesity, as a major risk for public health, has caused increasingly demand for anti-obesity supplements worldwide. Obesity can be a leading cause of many serious health problems, such as high blood pressure [1], cancer [2], type 2 diabetes [3], gallstones [4], heart diseases [5], stroke [6], erectile dysfunction [7], nonalcoholic fatty liver [8] and other health complications.
Management strategies for weight reduction in obese individuals include physical interventions such as exercise, diet, and surgery, behavioural therapies, and medicinal treatments. These strategies may be used alone or in combination for greater efficacy. Most randomized controlled trials (RCTs) evaluating pharmacotherapies include a calorie-controlled diet, and some also encourage participants to increase their physical activity.
Drugs used to induce weight loss may reduce appetite or increase satiety, reduce the absorption of nutrients, or increase energy expenditure. In the past drug therapies, available have included thyroid hormone, dinitrophenol and amphetamines, followed by amphetamine analogues, aminorex, and the fenfluramines [9]. More recently a number of newer agents have been trialled, though only orlistat was approved for long term use (≥24 weeks [9]. In fact, amphetamine, rimonabant and sibutramine licenses have been withdrawn due to an increased risk of psychiatric disorders and non-fatal myocardial infarction or stroke (Table1).
On the other hand, there is an increased tendency to alternative treatments that are mainly based on natural products or formulations for this problem. The increasing demand for natural slimming products can be easily understood mainly because of the false impression that, considering they are natural products, that do not cause either side effects or health damage. However, recent studies have demonstrated the presence of non-declared synthetic substances (i.e., adulterants) in the formulations of these so-called “natural products‟ worldwide [10,11].

The presence of synthetic substances, such as anorexics, in natural slimming formulations gives these products higher efficacy in the treatment of obesity. while the presence of these ingredients could not be discriminated due to misleading packaging. Unfortunately, continuous consumption of chemical slimming products, which are illegally adulterated with synthetic materials, may cause severe troubles to patients and could be considered as a threat to individual’s health and even a reason for mortality [12-14]
Most weigh loss adulterants are compounds that have been removed from the market by regulatory agencies or were never approved for use because the formulations are registered in compositions, or it is adulteration practice violates the laws of many countries. Adulteration caused a variety of adverse effects from mild (allergic reactions, fatigue, gastrointestinal upset, mood disturbances or muscle weakness, nausea, pain, and respiratory complaints) to moderate (confusion, convulsions, dermatitis, lethargy or seizures, leucopoenia, sensory disturbances, vomiting) to severe (carcinomas, cerebral oedema, coma, intracerebral haemorrhage, poisoning, metabolic acidosis, multi-organ failure, nephrotoxicity, perinatal stroke, renal or liver failure or death) life threatening effects see (Figure 1) [15] .
One notable example is the adulteration of a slimming product known as Slim 10 by N-nitroso fenfluramine, a hepatotoxic agent, possibly leading to a fatal case of hepatic failure in Singapore [16]. N-Nitroso fenfluramine was structurally modified from fenfluramine, a previously used anti-obesity drug. The authors concluded that in the absence of a more plausible cause of liver damage, and with nitrosoamines being known to be hepatotoxic, the likely cause of hepatocellular necrosis was the nitrosofenfluramine present. From 2001 to 2002, more than 800 cases of liver damage in Japan were reported among people taking Chinese weight loss aids containing nitrosofenfluramine [17]. In the subsequent year, three cases of severe hepatotoxicity associated with a N-nitrosofenfluramine-containing weight loss supplement were reported [18].
Most common adelterants in slimming products and associated problems
Amphetamines and amphetamine-like analogues
Amphetamines and amphetamine-like analogues (phentermine, diethylpropion, phenylpropanolamine) are indirect-acting sympathomimetic agents that act by releasing noradrenaline (NA) from presynaptic vesicles in the lateral hypothalamus [19]. Mazindol, a related but discontinued drug, blocks the reuptake of NA by presynaptic neurons. The increase in NA concentration within the synaptic cleft results in the stimulation of β2-adrenergic receptors and a resultant inhibition of appetite. Likewise, amphetamine and its derivatives had been used to treat obesity since 1937, though their addictive potential soon became obvious and they were removed from the market for this purpose. Amphetamine- like substances derived from the β-phenyl ethylamine core structure have been detected in dietary supplements [20].
Phentermine
Phentermine has been available since the late 1950s and is approved for short-term use in the US and Australia. It has been evaluated as both monotherapy and as combination therapy though not in large-scale studies [21]. Phentermine has been used in combination with fenfluramine and with fluoxetine Combination therapy with phentermine (15mg) fenfluramine (60mg), demonstrated significantly more weight loss than placebo in a 28-week RCT [22].
Diethylpropion (amfepramone)
Another amphetamine-like analogue has been available for weight loss since the early 1960s; however, there are few if any RCTs of its long term use especially with large sample sizes [23]. Diethylpropion (75mg daily) demonstrated significantly greater weight loss in a small 24-week study of 20 patients than placebo [24]. Diethylpropion (50mg twice a day) was shown to be more effective than placebo in a small 6-month RCT with 69 obese adult patients (9.3kg [95% CI 7-11.5kg] versus 3.1kg [95% CI 1.8-4.3kg] [25]. Greater than 5% weight loss was achieved in 67.6% of diethylpropion patients and 25.0% of those receiving placebo. The most common side effects were dry mouth and insomnia These were experienced in the first 3 months but become less apparent with continuing treatment [25].
Fenfluramines
Fenfluramine and dexfenfluramine elevate serum levels of serotonin (5HT) in the central nervous system by stimulating 5HT release and inhibiting its reuptake. Increased levels of 5HT appear to stimulate the hypothalamus, which controls satiation as well as mood, sleep, body temperature and other vital functions. These agents also activate melanocortin 4 receptors that in turn stimulate activation of 5-HT2C receptors, producing an increased release of 5HT within the hypothalamic-pituitaryadrenal axis which is claimed to lead to hypophagia and anorexia [26].
A meta-analysis of RCTs with fenfluramine and dexfenfluramine demonstrated higher weight loss than placebo following up to 12 months of treatment. The greatest efficacy was shown following 3 months treatment, 3.7kg weight loss, Although RCTs with fenfluramines (fenfluramine and dexfenfluramine), either alone [27] or with phentermine [22], demonstrated significant weight-loss, they were withdrawn from the market due to increased reports of valvular heart disease and primary pulmonary hypertension [28]. The prevalence rates of both valvular heart disease and primary pulmonary hypertension were higher following longer exposure to the fenfluramines [20].
Antidepressants
Fluoxetine
Fluoxetine, a selective serotonin reuptake inhibitor (SSRI) that augments 5HT within the central nervous system has been prescribed off-label for weight loss. Although significant weight loss was reported with 60mg of this agent in shortterm studies of 6-8 weeks, with maximum weight loss achieved at 12-20-weeks, this is followed by a regain in bodyweight [29]. Most RCTs have not shown a significant difference when fluoxetine was compared to placebo at 52 weeks [28]. Fluoxetine generally has a tolerable safety profile with reported adverse events of headache, asthenia, nausea, diarrhoea, somnolence, insomnia, nervousness, sweating, and tremor [29].
Bupropion
Is another antidepressant which inhibits reuptake of dopamine (DA) and NA resulting in a loss of appetite and decreased food intake and modest weight loss in obese people [30]. The efficacy of bupropion as a sustained release formulation was demonstrated at 48 weeks in obese patients. Weight loss was dose dependent with 7.5% initial weight loss for subjects Although bupropion is not approved for weight loss, it has been used off-label and is currently under evaluation as combination therapy with naltrexone, a μ-opioid receptor antagonist and zonisamide, a GABA receptor activator
Orlistat
Orlistat (a gastrointestinal lipase inhibitor) is a synthetic drug derived from a naturally occurring lipase inhibitor. It does not directly act on appetite as other obesity pharmacotherapies, rather it decreases fat absorption by binding to pancreatic lipase, the principle enzyme that hydrolyses triglyceride.
The long-term efficacy of orlistat (120mg three times daily) for weight loss has been demonstrated in several RCTs of 2- to 4- year therapy compared to placebo [31], as well as improvements in blood pressure, insulin resistance, and serum lipid levels [32].
Several systematic reviews in adults and a systematic review with 2 short-term studies in adolescents demonstrated significantly more weight loss with orlistat than placebo, 6.2kg [32]. The most commonly experienced side effects of orlistat are gastrointestinal and include diarrhea, flatulence, bloating, abdominal pain, and dyspepsia) [32].
Sibutramine
Sibutramine, a 5HT and NA uptake inhibitor, was originally developed as an antidepressant and subsequently found to reduce appetite) [33]. It has 2 active metabolites, which inhibit NA and 5HT uptake (and to a lesser extent DA) without any direct effect on neuronal NA, DA and 5HT release. It has been suggested that sibutramine has a dual action to facilitate weight loss, an anorectic effect suggested to be mediated through the central α1 and β1 adrenergic receptors and thermogenic effects through β3 adrenergic receptors peripherally) [34]. Maximal weight loss occurs by 6 months with sibutramine treatment and was dose related) [35].
Apart from increases in BP and heart rate the most common side-effects reported with sibutramine are dry mouth, constipation, and headache [33] associated with a higher rate of CV events than placebo whilst data from a FDA early communication indicated that there was an increased rate of CV events (heart attacks, strokes, resuscitated cardiac arrest, CV death) in patients with cardiovascular disease and diabetes. The EMEA concluded that the benefits of sibutramine did not outweigh the risks and recommended that all marketing authorisations for medicines containing sibutramine should be suspended throughout Europe. In 2010, however, the use of sibutramine was banned because of an increase in the relative risk for major adverse cardiac events in elderly overweight and obese patients.
Rimonabant
Rimonabant, an endocannabinoid receptor (subtype 1) blocker, was developed as a result of observations on the appetite stimulation associated with recreational cannabis use. The drug has a range of both central and metabolic peripheral effects and had also been investigated for smoking cessation [34].
Attrition rates in a pooled study of 5,580 patients without diabetes and 1,047 patients with diabetes taking rimonabant 20mg daily for one year and a hypocaloric diet were approximately 40% [36].
Phenolphthalein
Phenolphthalein had been used as an over-the-counter laxative and has often been detected together with sibutramine in adulterated weight-loss supplements products. After finding the potential carcinogenicity of phenolphthalein, it was reclassified in 1997 as unsafe and ineffective [37].
Analytical methods for the determination of adulterants in phytotherapeutic formulations
The development of analytical methodologies to selectively identify synthetic substances as adulterants in phytotherapeutic formulations is of great relevance from either a clinical or toxicological point of view.
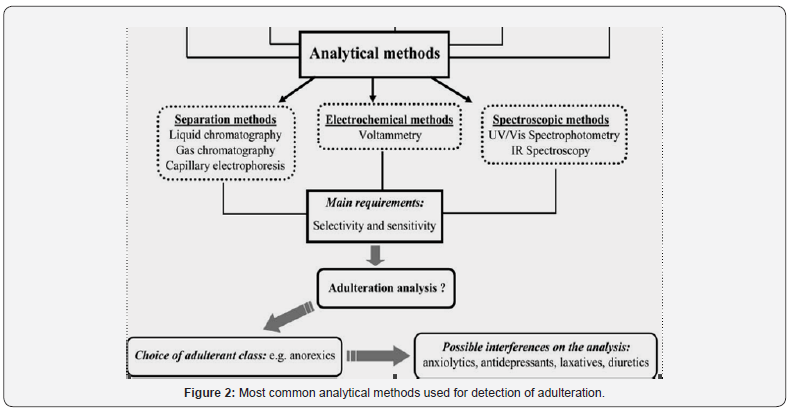
Toward this end, research involving the development of new methodologies for this purpose should first select the compound classes that would probably appear as synthetic pharmaceuticals in slimming formulations.
After identifying these possible adulterant classes, the most frequently used pharmaceuticals in each selected class (e.g., anorexics) should then be followed through individual studies, including their possible pharmaceutical associations (e.g., anorexics plus anxiolytics).
Figure 2 described below shows most common adulteration methods involving different pharmaceutical classes. The classification described in this scheme is based on the adulteration cases already reported in the literature, involving the most probable adulterant classes used in slimming formulations. It is apparent that a systematic study regarding possible adulterant classes can restrict analytes of interest by analytical method. The main requirements that must be fulfilled by the chosen analytical method are selectivity and sensitivity, as the possibility for adulteration is very wide among the probable classes. Once the adulterant class has been selected, all other probable adulterants become possible interferents in the analytical measurement using the selected method. Furthermore, these interferents should be systematically investigated to assure the applicability of the method to real samples, where there is a certain unpredictability regarding the type and class of adulterant to be found.
Chromatographic and electrophoretic methods
Most studies reporting on the adulteration of phytotherapeutic formulations apply chromatographic methods to determine and identify the adulterants.
These methods can be applied to complex mixtures, as they have a high separation capacity. This is an important feature for the analysis of phytotherapeutic formulations, which may not only have natural constituents but also synthetic adulterants. Additionally, HPLC is a well-established and widespread technique for routine analysis all over the world. Here, the detection by coupled mass spectrometry (LC-MS or LC-MS/MS) and nuclear magnetic resonance (LC-NMR) is very advantageous due to the possibility of adulterant confirmation based on structural information [35].
Among the chromatographic methods, gas chromatography (GC) has been also frequently used for the determination of adulterants in phytotherapeutics, where GC-MS is the most used technique, mainly for the determination of benzodiazepines, anorexics and stimulants [36].
Furthermore, GC-MS has been the method of choice for the analytical screening of these adulterant classes. Most of the described methods are developed for the screening and the confirmation of the adulterants based on their specific retention times and some additional spectral information [37] as well. The adulterant analysis involves often the quantification and confirmation of the pharmaceuticals by using the addition of high purity reference standards [38].
Capillary electrophoresis (CE) is an analytical technique that is becoming important among the separation techniques available for the determination of adulterants in phytotherapeutics. Some advantages of CE, such as high-resolution power, short analysis time, and low consumption of chemicals and samples, make it an attractive method for this kind of investigation [39].
Electrochemical and spectroscopic methods
Among the electrochemical methods, voltammetry is the most often utilized technique for the analysis of phytotherapeutic adulteration [40].
Voltammetric methods enable the sensitive and selective measurement of organic compounds based on their specific electrochemical behavior at the working electrode surface [20]. Electrochemical methods are advantageous in comparison with separation methods (HPLC and CE). This is because they allow the measurement of a sample without the total solubilization and exhaustive filtration steps that are necessary for the separation of insoluble excipients, as for HPLC and CE [40].
Furthermore, voltammetric methods are advantageous with respect to the low cost of instrumentation and the short analysis time [41]. De Carvalho et al. [41] used electrochemical methods to determine amfepramone, the most consumed anorexic all over the world, in synthetic mixtures containing other anorexics, benzodiazepines, and antidepressants [41].
Other relevant pharmaceuticals as adulterants of phytotherapeutics were systematically investigated by Correia [40] using the stripping voltammetry. The selective determination of seven benzodiazepines (clonazepam, flurazepam, alprazolam, midazolam, diazepam, medazepam, chlordiazepoxide) was shown to be possible in the presence of other adulterant classes (anorexics, antidepressants and hypoglicemics) in different phytotherapeutic formulations. This methodology permitted the rapid screening of the samples concerning the presence of benzodiazepines as adulterants. A simultaneous determination of benzodiazepines (clonazepam, bromazepam, midazolam, diaze- pam, medazepam, and flurazepam) was also investigated by dos Santos et al. [42] by stripping voltammetry, which also permitted the selective determination of these pharmaceuticals in phy- totherapeutic samples. Among the methods applied for the analysis of adulterants in phytotherapeutics, spectroscopybased techniques are used less frequently [43].
Despite their simplicity and low cost in routine analysis, these methods suffer serious interferences from complex matrices, such as the herbal formulations. Thus, time-consuming pre-treatment steps are normally necessary to eliminate the matrix interferents [44]. The pre-treatment of phytotherapeutic samples prior to the electrochemical and spectroscopic analysis involves normally the extraction of the adulterants by using methanol, ethanol, and chloroformas organic solvents [45].
The aforementioned electrochemical and spectroscopic methods permit the rapid screening of adulterants in phytotherapeutic formulations. The selective determination of pharmaceuticals based on their specific electrochemical behavior at electrode surfaces can be also useful as an additional confirmation method for adulterants. However, the spectroscopic methods (IR and UV/ vis) work mostly as a screening method for adulterants, since various organic components of the sample can absorb the electromagnetic radiation in the same wavelength regions than the most common adulterants.
Conclusion
The consumption of weight-loss formulations has increased markedly in the past few years. Both synthetic and natural products represent these formulations. The identification of controlled substances, which have been illegally added to phytotherapeutics, is a concern to regulatory bodies, as well as to consumers. The adulteration cases already reported in the literature refer mainly to the use of anorexics, benzodiazepines, and antidepressants. The increasing interest into the identification of these frauds, mainly due to the risks of adulterant interactions in the human body, leads us to predict that many cases will be uncovered in the future. In this context, sensitive and selective analytical methods have been developed. The main adulteration cases have been reported in Asia and Africa due to low quality control measures. There are still no officially established regulations by governmental organizations for the control of phytotherapeutics, and the adulteration of these formulations are a recurrent practice all over the world. The adulterant classes reported on here are the most frequently cited in adulteration cases related to formulations that are commercialized for slimming purposes.
To Know more about Pharmacy & Pharmaceutical Sciences
Click here: https://juniperpublishers.com/index.php




Excellent information on your blog, thank you for taking the time to share with us. Amazing insight you have on this, it's nice to find a website that details so much information about different artists.
ReplyDeletenatural slimming products