Cardiology & Cardiovascular Therapy - Juniper Publishers
Abstract
Amyloidosis forms a set of diseases characterized histologically by extracellular deposition of insoluble proteins with fibrillar conformation in the tissues. In diffuse forms, heart, kidney and digestive tract are preferentially affected, more than the skin and the peripheral nervous system. Three types of amyloidosis predominate: AL that contains immunoglobulin light-chains, AA or serum amyloid A protein, and transthyretin amyloidosis (ATTR). We report the case of a 65-year-old male hospitalized for Acute Coronary Syndrome and in whom clinical investigations will find non-AA type amyloidosis. Amyloidosis AL is the commonest type, and is associated with cardiac involvement of variable extent in almost 70% of the cases. The diagnosis can be challenging since echocardiographic and cardiac MR findings are nonspecific. Coronary involvement may be due to the presence of infiltration of amyloid deposits at the vascular level. However, only a biopsy of a coronary vascular fragment can confirm the diagnosis with the contribution of histology but this can only be done in post mortem. Cardiac amyloidosis is a rare condition with a poor prognosis, the diagnosis of which should be made early. Coronary artery disease should be systematically sought by cardiac catheterization, in order to improve management.
Keywords: Amyloidisis; Acute coronary syndrome
Introduction
Amyloidosis forms a set of diseases characterized histologically by extracellular deposition of insoluble proteins with fibrillar conformation in the tissues. In diffuse forms, heart, kidney and digestive tract are preferentially affected, more than the skin and the peripheral nervous system. Three types of amyloidosis predominate [1]: AL that contains immunoglobulin light-chains, AA or serum amyloid A protein, and transthyretin amyloidosis (ATTR). Cardiac involvement is mainly manifested by hypertrophic cardiomyopathy or heart failure with preserved ejection fraction (HEFPeF) more rarely by coronary artery disease. We report the case of a 65-year-old male hospitalized in the Cardiology Department of Ibn Rochd Universitary Hospital of Casablanca, Morocco, for Acute Coronary Syndrome and in whom clinical investigations will find non AA type amyloidosis.
Case Report
A 65-year-old male patient, chronic smoker at 40 packs/year, was admitted to our cardiology department for chest pain, a typical angina suggesting an acute coronary syndrome complicated by heart failure.
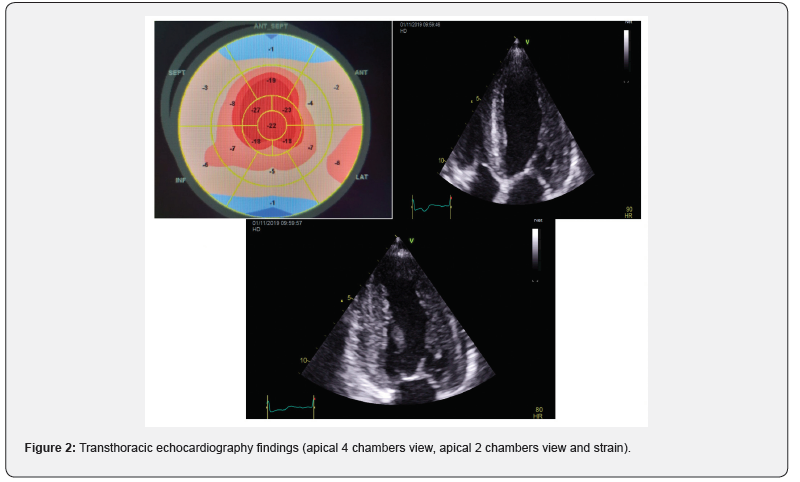
Upon admission, the patient presented typical anginal chest pain with class III NYHA dyspnea, at his clinical examination, his blood pressure was at 130 / 80 mmHg, heart rate at 80 bpm. He presented a global cardiac decompensation, with right heart failure signs that were predominant: retro malleolar edema, ascites of great abundance, turgor of the jugulary veins and hepatic jugular reflux and crackles in both pulmonary bases. The interrogation found a notion of low abundance rectorragia and right hypochondrium pain. The EKG recorded a regular sinusal rhythm at 75, with extreme low voltage in the limb leads (all ,0.5 mV) with a pseudoinfarction pattern in the anterior leads (QS waves from V1 to V3) (Figure 1). Transthoracic ultrasound, noted a symmetrical concentric hypertrophy with a septal wall thickness at 15mm and a posterior wall thickness at 15mm and a discreet inferolateral and anterolateral hypokinesia with a left ventricular ejection fraction (LVEF) at 55%. A longitudinal systolic dysfunction (S’LVseptal = 4cm / S, S’LVlateral = 6cm / S) with alteration of the GLS (global longitudinal strain (-11.5) at the expense of the bases with conservation of the apical kinetics (appearance in roundel of apical spearing). A sparkling and granite appearance of the interventricular septum, without an intra Left ventricular (LV) gradient. Type II diastolic dysfunction; with grade I diastolic dysfunction, impaired relaxation (E / A = 0.7) with an increase in filling pressures (E / E’= 17). The left atrium was dilated to 37ml / m2. The right ventriculi was dilated and hypertrophied (right ventriculi lateral wall = 10mm), with a preserved longitudinal function and without pulmonary hypertension (systolic pulmonary artery pressure SPAP at 30mmHg) and a dilation of the Inferior vena cava to 22 mm compliant (Figure 2).

In the assessment, we noted positive ultrasensitive troponins at 3857ng/L, Pro BNP at 270 ng/L. The ultrasound aspect allowed us to evoke the diagnosis of a cardiac amyloidosis nevertheless in front of the anginal chest pain, the troponins positive we carried out a coronarography by radial way that objectified a long subacute occlusion of the proximal left anterior descending artery, recovered through the right network with a good downstream bed and a tight long stenosis of the proximal right coronary artery. With indication for medical treatment. In front of the digestive manifestations, and the rectorragia, an esogastroduodenal fibroscopy was indicated with biopsies objectifying a fundic and antral erythematous gastritis and a congestive bulbitis. The anatomopathological study showed a chronic pangastritis with presence of intestinal metaplasia without malignancy signs and red congo positive interstitial amyloid deposits, found also in the vascular walls, with phenotyping showing non-AA amyloidosis. The patient was put on treatment of his ischemic disease with double anti platelet aggregation, Aspirin 75mg per day, Clopidogrel 75mg per day, angiotensin-converting enzyme inhibitors 5mg per day, Beta-Blocker 2,5mg per day, Statins 10mg per day and Furosemide 40mg per day with good clinical progress, improvement of dyspnea and regression of chest pain, nevertheless he kept ascites in relation to his intestinal amyloidosis.
Discussion
The amyloid diseases are protein misfolding diseases in which the pathogenic protein misfolds and aggregates as insoluble amyloid fibrils, most commonly in the extracellular space. Three types of amyloidosis predominate [1]:
• AL amyloidosis, characterized by k or λ monotypic
deposits and associated with lymphoproliferation and especially
with monoclonal gammopathy.
• AA amyloidosis, occurring in a chronic inflammatory
context.
• Transthyretin amyloidosis (ATTR), which is either senile
or hereditary.
• Cardiac amyloidosis is a rare disease, which is
characterized by infiltration of insoluble proteins in cardiac,
muscular and / or vascular tissues. Coronary amyloidosis is still a
rarer entity, the diagnosis is confirmed post-mortem [2].
Amyloidosis AL is the commonest type, and is associated with cardiac involvement of variable extent in almost 70% of the cases. Cardiac amyloidosis has a poor prognosis, requiring early diagnosis [2,3]. The disease typically presents as a restrictive cardiomyopathy secondary to interstitial amyloid deposits; Hypertrophic cardiomyopathy, Heart failure with preserved ejection fraction (HEFPeF) however it can uncommonly present ischemic symptoms [4].
Extracardiac clinical signs should be sought on clinical
examination: carpal tunnel syndrome, macroglossia, periorbital
bruising, neuropathy. Electrocardiogram EKG can show micro voltage, conduction
disorder or pseudo-Q wave. Transthoracic ultrasound objective:
• Atrial septal thickening considered a characteristic
feature of cardiac amyloidosis,
• Characteristic granular/sparkling appearance of the left
ventricular (LV) myocardium: not specific and need to differentiate
from other infiltrative diseases,
• Increased Left Ventriculi wall thickness which results
from amyloid infiltration of interstitial space and may relate to
amyloid burden [5].
The biological assessment includes protein electrophoresis, immunofixation, determination of free immunoglobulin light chains, Bence Jones proteinuria, NT-pro BNP and troponin. The genetic analysis of TTR is performed when there is an argument for transthyretin amyloidosis. MRI (late enhancement of gadolinium) and scintigraphy (cardiac fixation of the tracer) anomalies are also very suggestive of cardiac amyloid involvement but the diagnosis of amyloidosis is purely anatomopathological [6,7]. The least invasive biopsies are performed as a first intention. If they are negative, a cardiac biopsy may be necessary to confirm the diagnosis [7]. A retrospective study involving histopathological review of myocardial tissue from 98 patients with AL amyloidosis showed that 66% patients had intramural amyloid deposits. However, only 25% of these patients had any symptoms suggestive of ischemia [8,9]. The extent of vascular involvement in cardiac amyloidosis is variable and depends on the type of amyloidosis [4].
The diagnosis can be challenging since echocardiographic and cardiac MR findings are nonspecific [4,10]. Likewise, the mild elevation left ventricular filling pressures, which may be noted in cardiac catheterization, are nonspecific. Coronary angiography is typically normal as the infiltration is predominantly microvascular [4]. Consequently, the diagnosis of this form of cardiac amyloid is often delayed. The cardiac localization of AA amyloidosis is rare and exceptionally symptomatic, whereas that of non-AA in particular AL is very frequent as it can reach 60% of cases [3,11]. The prognosis is serious with symptomatic cardiac involvement, the average survival is only a few months [12].Authors suggest that amyloid deposits can sometimes be confined to the coronary arteries [9]. On the other hand, several autopsy studies have been reported, among which, the study by Smith et al. which examined 47 hearts of patients with cardiac amyloidosis (21 primary and 26 senile) the vascular component a was found in 19 patients with primary amyloidosis and 1 patient with senile amyloidosis. Indeed, coronary amyloidosis was present in most of the patients (66%) with primary cardiac amyloidosis. Because only 3 patients had an isolated vascular attack. This study suggests that vascular involvement and ischemia are more important than previously thought [13]. Cardiac involvement of amyloid origin is therefore due to its protein infiltration into the cardiomyocytes, thereby achieving rigidity and thickening of the myocardium, most often causing restrictive cardiomyopathy evolving in three stages [8,12]. Coronary syndrome is also part of one of the entities of cardiac amyloidosis by infiltration of amyloid deposits in the various coronary vessels [7,9,12].
These various hypotheses evoking vascular damage of an ischemic nature in cardiac amyloidosis corroborate with our diagnosis on the possibility of coronary attack in our patient suffering from cardiac amyloidosis. Coronary involvement may be due to the presence of infiltration of amyloid deposits at the vascular level. However, only a biopsy of a coronary vascular fragment can confirm the diagnosis with the contribution of histology but this can only be done in post mortem [14].
Conclusion
Cardiac amyloidosis is a rare condition with a poor prognosis, the diagnosis of which should be made early. Coronary artery disease should be systematically sought by cardiac catheterization, in order to improve management.
To Know more about Cardiology & Cardiovascular Therapy




No comments:
Post a Comment