Journal of Toxicology - Juniper Publishers
Abstract
Hyperthyroidism is a common disorder in older cats
causing detrimental adverse effects if left untreated. The three most
recommended treatment options include thyroidectomy, radioiodine
treatment, and antithyroid medication therapy. Oral methimazole has been
the most widely used option due to low cost and accessibility. The
topical application of transdermal methimazole is an ideal route of
administration for cat owners. The purpose of this review article is to
give insight into the efficacy and recommended indication for use of the
pluronic lecithin organogel (PLO) formulated transdermal delivery
system of methimazole, in the treatment of feline hyperthyroidism. PLO
compounded methimazole is uniquely transported through the skin, and
chronic use has been shown effective in treating feline hyperthyroidism.
In many cases, once daily application of the gel has provided enough
methimazole activity for lowering hormone levels. The compounded
formulation also allows for more individualized dosing than the oral
tablets. There is limited information regarding long-term treatment of
PLO methimazole, however, the formulation continues to satisfy both
veterinarians and owners, and effectively lower serum thyroxine (T4)
concentrations.
Abbrevations: PLO: Pluronic Lecithin Organogel
Introduction
Over the past 20 years, the prevalence of feline
hyperthyroidism has increased astoundingly [1-3]. It has become the most
common endocrine disorder in cats, and the risk worsens with each year
of increasing age, being most common in middle to older-aged felines
[1]. The disease is primarily characterized by an excessive production
and release of the thyroid hormones thyroxine (T4) and triiodothyronine
(T3) most commonly due to a functional, benign adenomatous hyperplasia
of the thyroid gland. At present, there is not a feline specific thyroid
stimulating hormone (TSH) assay test available, therefore unlike human
hyperthyroid
diagnosis, veterinarians do not commonly depend on a low TSH value for
primary hyperthyroid diagnosis. Hyperthyroidism diagnosis in cats is
generally based on a high free T4 level and the presence of clinical
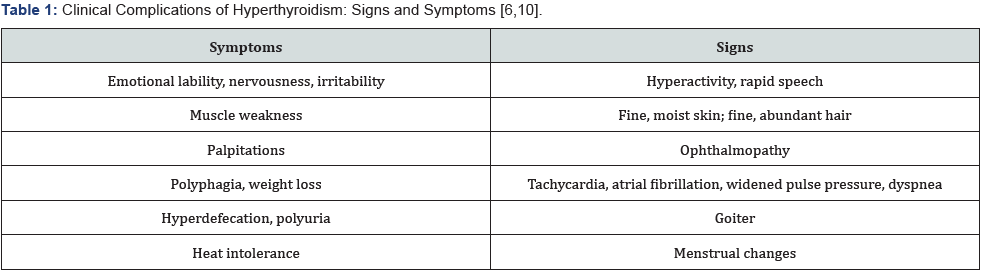
signs and symptoms. Some of the clinical complications of
hyperthyroidism that may be present include emotional lability,
hyperactivity, palpitations, tachycardia, and a plethora of other
manifestations of the disease (Table 1). Although the exact etiology is
unknown, many nutritional and environmental causes are suspected,
including canned cat food products containing iodine, soybean,
phthalates, polyphenols, and polychlorinated biphenyls [2,4,5].
Regardless of the etiologic origin, medical management of
prolonged thyroid hormone elevation is very important. Untreated
hyperthyroidism can have many consequences on the cat. Many
cats initially present with a change in personality or behavior, often
being more easily agitated and mean, as well as with unexplained
weight loss, changes in eating habits, accelerated heart rates,
and a goiter. Hyperthyroidism, if left untreated, can also have
life threatening adverse effects, such as causing hypertension,
cardiac tachyarrhythmia, atrial fibrillation, and even death [6,7].
These result from elevated thyroid hormone levels and cause
up-regulation of various gene expressions involved in the body’s
metabolism, thermogenesis for heat regulation, nerve function,
and muscle and bone function [7]. They also function to increase
activation of the sympathetic nervous system, which elevates the
heart rate, the heart’s force of contraction, and increases cardiac
output overall [8,9]. Clearly, both the symptoms of the disease,
as well as the enhancement of these biochemical pathways, can
pose serious health risks to the feline patient. The longer a cat
goes without treatment, the worse their complications become
[6,10,11].
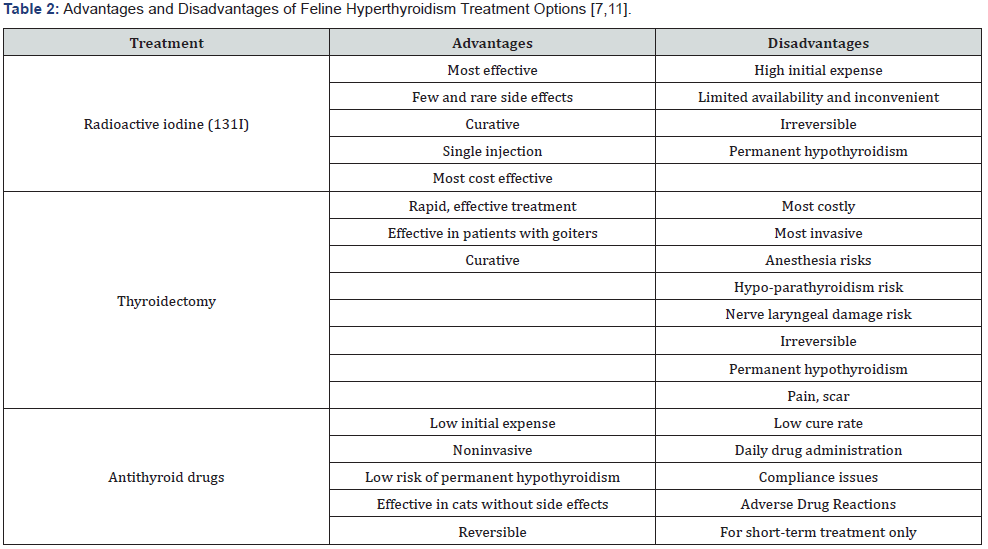
Like the management of hyperthyroidism in humans, there
are several different treatment options available for cats. The top
three recommended therapies include surgical thyroidectomy,
radioiodine therapy, and medication treatment. Thyroidectomy
and radioiodine treatment can be permanent solutions to the
disease. However, limitations such as expense and permanent
hypothyroidism prevent these from being practical options for
most feline patients [7,11] (Table 2). Medication therapy is often
the most practical and accessible way to manage hyperthyroid
cats. Methimazole (Tapazole, Felimazole) is the most common
and favored agent in the United States [12]. Other alternatives
include carbimazole (a prodrug of methimazole marketed only in
the UK), iodine-containing agents, iodine dietary restricted food,
and homeopathic regimens [6,12-14]. Dietary iodine restriction
is another option, however, there is limited supporting data to
determine a true benefit.
Although several treatment options are available for
hyperthyroid cats, each therapy option has considerable
drawbacks to both the client and the feline patient. Oral
methimazole has historically been the most accessible and
affordable choice. However, gastrointestinal side effects and
an unfavorable twice-a-day oral administration schedule often
limit its ultimate therapeutic outcomes in the cat. Both negative
attributes are avoided with use of the transdermal methimazole
gel compound. Due to the limited amount of data available on
transdermal methimazole, this review aims to evaluate whether
the pluronic lecithin organogel (PLO) compound of methimazole
is effective in treating hyperthyroid cats. In addition, it also serves
to provide insight on the recommendations for its use.
Methods
A PubMed search was conducted to identify articles
in which
the safety or efficacy of transdermal methimazole for treatment
of feline hyperthyroidism was assessed. Key MeSH search terms
included feline hyperthyroidism with a subheading for treatment.
In addition, feline hyperthyroidism plus one of the following
search terms were searched: treatment, drug-related side effects and
adverse drug reactions. A free-text search was also conducted
to identify articles not included in the MeSH term search. Metaanalyses,
randomized controlled clinical trials, and case reports
were included in the review if the primary focus of the article
related to the use of oral or transdermal methimazole for feline
hyperthyroidism. Studies were excluded if published in languages
other than English. In addition, studies highlighting mechanisms
of action, studies of pharmacodynamics or pharmacokinetic
effects were excluded.
Results
Clinical data on the topic of feline hyperthyroidism treatment
is limited. A PubMed search revealed 14 articles with transdermal
methimazole and feline hyperthyroidism as a subheading. Of the
articles used in this review, there were six that directly assessed
the use and efficacy of transdermal methimazole in the treatment
of feline hyperthyroidism. Of those six, five were small clinical
studies and one was a case report/series.
Evaluation of oral methimazole
Oral methimazole has remained the mainstay of feline
hyperthyroidism treatment since the early 1980’s. It reversibly
suppresses thyroid hormone levels by inhibiting thyroid
peroxidase. It does not inactivate circulating T4 and T3, resulting
in a 2 to 4-week delay before serum T4 concentrations begin
to normalize [8]. While it accumulates in the thyroid gland, it
does not block the release of preformed hormone, nor does
it help reduce goiters [8,15]. Oral methimazole has variable
bioavailability ranging from 27 to 100% so its efficacy varies
from patient to patient [6]. The recommended dose for maximum
efficacy is 2.5mg administered twice daily.
In a randomized, unblinded, clinical trial by Trepanier et
al. [11], forty methimazole naive cats with newly diagnosed
hyperthyroidism were studied to compare the efficacy of one
daily dosing of oral methimazole to twice daily dosing. Owners
completed a questionnaire of their cat’s baseline behavior status
and reported any changes that occurred during the study. The
overall efficacy of once daily methimazole was found to be less
effective than twice daily dosing. Serum T4 concentrations were
considerably higher in cats receiving once daily dosing, and only
54% (13/24) were found to be euthyroid at two weeks, compared
to 87% (13/15) euthyroid in the twice daily group [16]. Both
treatment groups showed considerable clinical improvement
of many complications caused by hyperthyroidism. However,
among the initial 40 cats studied, one cat in the once daily dosing
group was removed prior to the 2-week point due to considerable
gastrointestinal (GI) upset. Of the remaining 38 feline patients,
17 (44%) developed some type of adverse event throughout the
four-week duration. Throughout the remainder of the study, 23%
(9 cats) reported similar GI upset. Among the 24 cats treated
once daily, 42% (10/24) required discontinuation of therapy,
in order to resolve oral methimazole induced adverse events.
Facial excoriation was reported in six patients, five reported
from the once daily dosed group alone. Five of the six total facial
excoriation cases reported were from the once daily dosed group.
Manifestations of blood dyscrasias and hepatopathy were not
significantly reported in either group [16].
Not only were adverse events such as GI upset and facial
excoriations, found to be less prevalent in cats dosed twice a
day, but also these cats were also more likely to obtain the goal
euthyroid state. Cats also show rebound increases in serum T4
concentrations and a return to hyperthyroid state within 24 to
48 hours of methimazole discontinuation [3,16,17]. This likely
correlates with the need for twice daily dosing in cats, and further
research should be performed to help determine methimazole’s
true intrathyroidal residence time in cats. Oral methimazole is
not a cure for feline hyperthyroidism, and treatment must be
continued indefinitely. With the intolerable GI upset from the
oral tablets and the difficulty many owners face administering
the medication twice daily to uncooperative cats, the alternative
transdermal route of administration poses significant benefits
[16].
Transdermal methimazole formulation
Despite the limited clinical studies on transdermal
methimazole, some clinicians have achieved a good therapeutic
benefit to using this dosage form in cats. Pluronic lecithin
organogel is a microemulsion-based gel containing lecithin,
isopropyl palmitate, and pluronic acid to effectively deliver both
hydrophilic and lipophilic drugs topically across the stratum
corneum and may aid in the administration of methimazole [18-
22]. PLO is composed of both an oil phase (lecithin phase) and an
aqueous phase (pluronic phase). It includes isopropyl palmitate
acts as a solvent and permeation enhancer while lecithin also
serves as a permeation enhancer by increasing the fluidity of the
stratum corneum, and slightly disorganizing the skin structure
to permit substance permeation [23-25]. PLO reversibly turns
into a thick gel at body temperature, leading to an increase in
dehydration of the aqueous solution, forming a shell-like structure
of aggregated micelles [7,24-28]. Methimazole is an ideal drug for
transdermal delivery due to its low molecular weight, high lipid
solubility, water solubility, low daily dose, and is non-irritating
and non-sensitizing to the skin [20,24].
Efficacy of the PLO methimazole
In a small retrospective study examining dispensing records
for 16 hyperthyroid cats undergoing transdermal methimazole
treatment, the transdermal formulation was effective at reducing
serum T4 concentrations in 15 of the 16 cats studied. One cat
showed an increase in serum T4 level, but there is no mention
or clarification of appropriate application or other possible
contributing factors. The only adverse event reported was a single
case of increased blood urea nitrogen level, thought to be the
unmasking of prior renal disease. This study also demonstrates
variability in dosing and administration frequency of the topical,
ranging between 5 mg once a day to a twice daily dose of 7.5mg every morning and 5 mg every night. This wide variation between
each feline patient, limits our ability to recommend a standard
dose or administration frequency, but does indicate the need for
patient-specific doses and frequencies in order to effectively reach
the euthyroid goal [29].
In a randomized clinical trial conducted by Sartor et al, 47
newly diagnosed hyperthyroid cats were used to investigate
whether PLO formulated transdermal methimazole was safe and
efficacious in controlling feline hyperthyroidism. At two weeks of
treatment, more cats in the oral methimazole group had serum
T4 concentrations within the reference range (14 of 16 [88%],
p=0.035). By week four, there was no difference between the oral
and transdermal methimazole. The PLO transdermal methimazole
group took longer to reduce serum T4 concentrations to the
acceptable reference range, however, it was as effective as oral
administration in producing euthyroidism by the fourth week of
treatment [30]. Fewer GI adverse events were reported with the
transdermal formulation (1/27 vs 4/17 in the oral group). The
reduction of GI upset deems consideration as it is often the cause
of discontinuation of oral methimazole [30,31].
Lecuyer et al evaluated the efficacy of transdermal
methimazole in 13 newly diagnosed hyperthyroid cats. The feline
patients received 5mg methimazole concentrated in PLO, applied
to the inner ear twice daily. In addition to reaching the euthyroid
state, all 10 cats that completed the study also showed improved
clinical signs related to hyperthyroidism consistent with other
previously reported studies [16,32-33]. No GI adverse events
were reported, and investigators concluded that PLO transdermal
methimazole is a safe and effective alternative to oral methimazole
[6].
Duration of t4 suppression
A study by Boretti et al. [33] evaluated the duration of
serum T4 suppression among newly diagnosed hyperthyroid
cats treated with once daily transdermal methimazole versus
twice daily dosing. Twenty cats were treated with the PLO-based
methimazole formulation dosed either 2.5mg every 12 hours (10
cats, group 1) or 5mg every 24 hours (10 cats, group 2). Serum
T4 concentrations were measured one and three weeks after
initiation of therapy, immediately before and every two hours
after gel application for up to 10 hours. Cats were limited to a
maximum of five blood samplings in one day [33]. A sustained
suppression of T4 concentration for at least 24 hours was seen
following gel application and there was no significant difference
in change in serum T4 concentration immediately before or any
time after gel administration in either group. As also discussed
in Lecuyer’s study [6], further research is needed concerning the
duration of intra thyroid methimazole accumulation [6,33,34].
Among the twice daily dosing group, reductions were required in
three cats, and a dose increase was required in one patient. Of the
once daily dosing group, two cats required a decrease in dose, and
one cat required an increased dose, after three weeks of treatment
as a result of sustained hyperthyroid levels [33]. Investigators
concluded that once daily application of the PLO methimazole
compound can effectively reduce serum T4 concentrations in
most hyperthyroid cats. Once a day dosing is most convenient
for the owner, and thus promotes better compliance [33]. The
compounding of this preparation allows for changes in dose or
frequency and allows for the individualization of therapy.
PLO vs. novel lipophilic base
In a 12-week prospective study by Hill et al, a novel lipophilic
formulation of methimazole was investigated. The study
included 45 cats newly diagnosed with untreated, naturally
occurring hyperthyroidism [12]. The study used a novel lipophilic
formulation prepared with methimazole, “carrier compounds”
(propylene glycol, polyethylene glycol 4000, dimethyl formamide,
and cyclodextrin), and several penetration enhancers, chosen
from fatty acids, terpenes, pyrrolidones, a short chain alcohol,
glycol ethers, acetins, and triglycerides. The formulation was
determined to be stable for 12 months after preparation, by
the International Cooperation on Harmonization of Technical
Requirements for Registration of Veterinary Products. Cats were
treated with a starting dose of either oral carbimazole (5mg twice
a day) or the novel transdermal methimazole formulation (10mg,
or 0.1mL applied to the inner ear once a day). Both the once daily
novel transdermal methimazole and twice daily oral carbimazole
were effective in the treatment of feline hyperthyroidism in
cats with compliant owners. All owners were satisfied with the
improved clinical symptoms.
The novel lipophilic transdermal formulation had several
advantages over the oral carbimazole, as the transdermal
medication was tolerated better, and caused no gastrointestinal
side effects in the cats. Owners reported that administering
tablets to their cats was a challenge, and 35% admitted to missing
doses or cats spitting out the medication [12]. Unlike the rare
occurrences of pruritus reported with the PLO formulation of
methimazole, no adverse events of pruritus or erythema of the
inner ear were reported [6,12]. The study suggests that since
methimazole is a lipophilic drug, a lipophilic vehicle might more
suitable than the PLO base. Although this study clearly highlights the effectiveness of
once a day use of this novel lipophilic formulation, it would have
been more appropriate to study it in comparison with the PLO
methimazole formulated topical. The novel lipophilic formulation
appears to be less irritating to the skin among cats than the PLO.
However, this has not been shown clinically significant in any
study, and thus does not provide enough evidence to recommend
one transdermal formulation over the other [6,12,33]. Further
evaluation and study are needed to compare the costs, efficacy,
stability, accessibility, and adverse event rates between the PLO
and novel lipophilic formulations of methimazole.
Discussion
Transdermal drug delivery is an appealing route of
administration for veterinary medicine, especially for clients with
uncooperative pets. PLO used for methimazole is recognized as
a viable transdermal delivery tool because of its enhanced drug
transport capabilities. It can effectively deliver both hydrophilic
and lipophilic drugs. Transdermal methimazole circumvents the
liver’s first pass metabolism, potentially allowing a lower drug
dose for an equal effect while also avoiding the intolerable GI upset
often caused by oral drugs leading to discontinuation. Following
chronic daily application of PLO formulated methimazole to the
inner ear of cats with hyperthyroidism, successful resolution of
clinical signs and lower T4 levels have been noted [6,18,30,31,33].
Although ultimately effective, delayed onset of action was noted
and transdermal methimazole takes longer to achieve therapeutic
serum T4 concentrations compared to oral methimazole activity.
Oral administration may be more suitable in cats with very
severe hyperthyroidism, requiring rapid reduction of thyroid
hormone levels. Repeated dosing with the PLO formulation can
lead to exfoliation of the inner ear, mild inflammation, and may
cause a depot of drug in the skin [30,35]. As the PLO works to
compromise the skin barrier over time, more drug is absorbed.
Therefore, maximum effectiveness is not seen immediately, but
most feline patients will reach a euthyroid level by week 4 of
treatment. Transdermal methimazole can be deemed noninferior
to the widely approved oral formulation.
Oral methimazole has only been proven effective if dosed twice
a day in cats [16]. Once daily dosing of transdermal methimazole
was successful, however, the need for twice daily dosing was
recognized early in treatment. Once daily dosing presents an
obvious advantage as it is most convenient for the owner and
aids in promoting good compliance. Near perfect compliance
is imperative when treating hyperthyroidism, because serum
T4 concentrations can return to their hyperthyroid level within
48 hours after the last dose. Another unique advantage of the
transdermal formulation is that it can be compounded into any
dosage concentration needed.
In the past, transdermal methimazole was recommended only
for short-term use in cases of oral methimazole induced GI upset
or an uncooperative cat. Oral methimazole was indirectly favored
due to the cost, variable stability, and unknown pharmacokinetic
information of the transdermal form. However, more recent
studies have suggested extended effectiveness with long-term
use of the transdermal methimazole. Also, upon diagnosis of
hyperthyroidism, most cats are near the end of the life and shortterm
treatment is usually enough in resolving the hyperthyroid
illness until the cat expires due to other unrelated diseases.
Although the transdermal formulation is more expensive, it is still
a more reasonable cost compared to the expense of thyroidectomy
and radioactive therapy. Cat owners reported missing oral doses
or cats spitting tablets, thus the transdermal gel may be worth
the extra cost in order to manage the disease. Clients at large
reported satisfaction with the compounded medicine, with only a
few reports of precipitation of the gel [6].
Conclusion
Transdermal use of PLO compounded methimazole is an
effective therapy for lowering serum T4 concentrations in cats.
It is safe, posing fewer adverse effects than the oral formulation.
It can be effectively used to treat feline hyperthyroidism through
individualized dosing and frequency of administration. Owners
should rotate ears each application and remove any residue with
a damp cotton ball prior to the next application. Cats tolerate it
very well, and it is favored by owners for its convenience and
resolved GI upset events. Frequent monitoring of the cat’s liver
function tests, BUN, creatinine, CBC, platelet count, and serum T4
concentration is recommended. Very little data exists regarding
its pharmacokinetic properties and formulation stability, and the
significance of the information available is limited by the small
sample sizes studied.
To Know more about our Juniper Publishers
Click here: https://juniperpublishers.com/index.php




No comments:
Post a Comment