Journal of Organic & Medicinal Chemistry-Juniper Publishers
Abstract
Simple, sensitive and accurate UV-spectroscopic methods were developed and validated for simultaneous estimation of aliskiren and amlodipine in tablet formulation using simultaneous equation and first derivative spectroscopic methods. Developed methods include direct estimation of amlodipine at 365nm without any interference, since aliskiren has zero absorbance at this wavelength. Estimation of aliskiren through simultaneous equation was performed at 279 nm, while 236.8 nm were selected as zero crossing point for estimation of aliskiren by first order derivative spectroscopic method. Linearity was found to be satisfactory over the concentration range of 25-300μg/ml and 5-100μg/ml for aliskiren and amlodipine respectively. The mean percentage label claim of aliskiren and amlodipine using simultaneous equation was 99.84 and 99.85 % respectively, while for first derivative spectroscopic method it was found to be 100.36 and 99.85% respectively. The developed methods are economical and reproducible for routine analysis of aliskiren and amlodipine in tablet formulation.
Keywords: Aliskiren; Amlodipine; First order derivative method; Simultaneous equation Method; Validation
Introduction
Chemically aliskiren (ALS) is (2S, 4S, 5S, 7S)-5-Amino-N-(3- amino-2, 2-dimethyl-3-oxopropyl)-4-hydroxy-7-[[4-methoxy- 3-(3-methoxypropoxy)phenyl]methyl]-8-methyl-2-propan-2- ylnonanamide [1]. It is a is a white to slightly yellowish crystalline powder. Aliskiren is the first in a class of drugs called direct renin inhibitors. It is used for essential (primary) hypertension [2]. It is highly soluble in water, ethanol and DMSO [3,4]. Amlodipine (AML) is chemically 3-Ethyl-5-methyl (±)-2-[(2-aminoethoxy) methyl]-4-(2- chlorophenyl)-1, 4-dihydro-6-methyl-3,5- pyridinedicarboxylate. Amlodipine besylate is white to off white powder, crystalline and has long-acting 1, 4-dihydropyridine calcium channel blocker [5,6]. It acts primarily on vascular smooth muscle cells by stabilizing voltage-gated L-type calcium channels in their inactive conformation. By inhibiting the influx of calcium in smooth muscle cells, amlodipine prevents calcium-dependent myocyte contraction and vasoconstriction. Amlodipine is used to treat hypertension and chronic stable angina [7,8]. Several analytical methods have been reported for estimation of ALS and its combination with other drugs which includes spectrophotometry and HPLC [9-13]. Similarly, various spectrophotometric and HPLC methods have been reported for estimation of AML and its combination with other drugs [14-18]. In the present work, a successful attempt has been made to estimate both these drugs simultaneously using dual wavelength UV spectrophotometric method. Structures of both the drugs ALS and AML are given in (Figures 1 & 2).
Materials and Methods
Instrumentation
A double beam UV spectrophotometer (UV-1800, Shimadzu, Japan) with UV probe software version (2.31) and 10mm quartz cells was used. All weights
Reagents and Chemicals
Pure drug, Aliskiren hemifumarate and amlodipine besylate was procured from Swapnroop Drugs and Pharmaceuticals, Aurangabad, Maharashtra, India. Marketed formulation was procured from local Pharmacy. All the chemicals and reagents used were of A.R. grade.
Method Development
Preparation of Standard Stock Solution: The standard stock solutions of Aliskiren and Amlodipine were prepared by dissolving 110.5mg of aliskiren hemifumarate (110.5mg of aliskiren hemifumarate is equivalent to 100mg of aliskiren) and by dissolving pure drug of amlodipine besylate equivalent to 100mg of amlodipine in separate 100 mL volumetric flask containing sufficient quantity of distilled water, the solutions were sonicated for 5 min then volume was made up to the mark with distilled water to get a concentration of 1000μg/mL of each solution. The standard stock solutions were further diluted to obtain desired concentrations.
Preparation of Sample Solution: Twenty tablets were weighed and powdered. The quantity of the powder equivalent to 150mg of ALS was transferred to 100 ml volumetric flask. The content was mixed with sufficient quantity of distilled water and sonicated for 20min to dissolve the drug. The solution was then filtered through a Whatman filter paper no. 41 and made up to the mark with distilled water An aliquot of solution (1.0ml) was transferred to a 10 ml volumetric flask and the volume was adjusted up to the mark with distilled water to obtain required concentration of ALS (150μg/ml) and AML (10μg/ml).
Simultaneous Equation Method
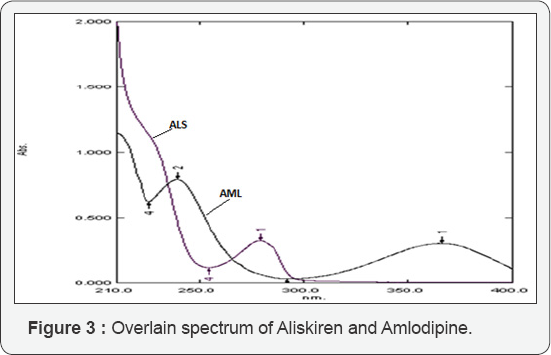
For simultaneous estimation of ALS and AML using simultaneous equation method (SE method) the solutions of ALS (50μg/ml) and AML (20μg/ml) were prepared from the standard stock solutions of ALS and AML and scanned over the range of 200 nm to 400 nm. An overlain spectrum was studied for development of suitable method for analysis. The overlain spectrum of ALS and AML is shown in (Figure 3). From the overlay spectra, 279 nm wave length was selected for the estimation of ALS using simultaneous equation method. Estimation of AML was done as a single component at 365nm. The absorptivity values were calculated and were applied in framed simultaneous equation 1, which is presented as,]

Where, A is absorbance of sample solution at 279 nm, CX and CY are concentrations of ALS and AML, respectively in μg/ml.
First Order Derivative Method
Estimation of AML was performed similarly as in simultaneous equation method. For estimation of ALS first order derivative method (DR method) was applied. The zero order spectrum was then derivatised to obtain first order derivative spectrum (Figure 4). From this spectrum of ALS and AML zero crossing point of 236.8 nm was selected using 2 nm as wavelength interval (Δλ = 2) and scaling factor taken as 1 for estimation of ALS.

Analysis of ALS & AML in Tablet Formulation
The absorbance of final sample solution was measured against distilled water as blank at 279 nm for SE method and at 236.8nm for DR method while the estimation of AML was done directly at 365nm. The analysis procedure was repeated five times for marketed formulation.
Method Validation
Linearity and Range: Aliquots of standard solution of ALS (0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 2.0 and 3.0 ml) and AML (0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6 and 1.0 mL) were transferred in a series of 10 ml volumetric flasks. The volume was adjusted up to the mark with distilled water and mixed. Absorbance values were recorded at 279 nm for SE method and at 236.8 nm for DR method against distilled water as blank for determination of ALS. Absorbance values of AML were recorded at 365 nm for all dilutions. The calibration curves were plotted between the concentration of component and absorbance values of ALS for SE method and between concentration and dA/dX for DR method. Calibration curve for AML was plotted between the concentration of component and absorbance value of AML.
Standardization of the Method by Analysis of Mixed Standard Solutions: To check the validity of the selected methods, mixed standard solutions of ALS and AML were prepared. The solutions were subjected to determine absorbance values at respective wavelengths and concentration of the components were calculated.
Accuracy: The accuracy of the method was determined for both the methods by calculating recoveries of ALS and AML by the standard addition method. Known amount of standard solution of ALS and AML were added at 80%, 100% and 120% levels to pre-quantified tablet sample solutions of ALS and AML. The results are reported in terms of % Recovery
Results and Discussion
Method Development and Validation
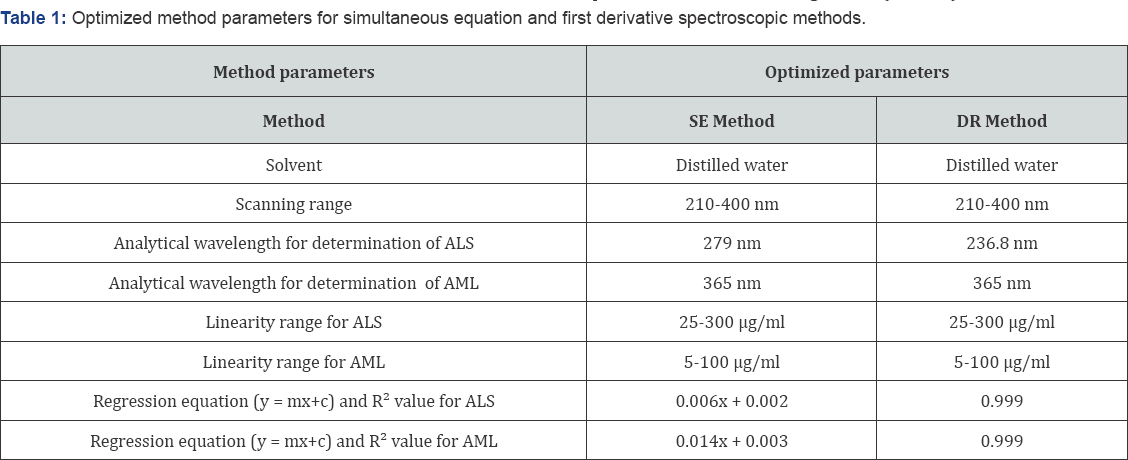
Two simple, sensitive and accurate UV-spectroscopic methods were developed and validated for simultaneous estimation of aliskiren and amlodipine in tablet formulation using SE and DR spectroscopic methods. From the overlain spectra of the drugs it was observed that SE and DR spectroscopic methods were suitable methods for simultaneous determination of ALS and AML. Distilled water was taken as solvent system, as both the drugs were soluble in this solvent and reduce the cost of the method. In SE method and DR method, wavelengths 279 nm and 236.8 nm respectively were selected for determination of ALS, whereas AML was estimated directly at 365 nm as ALS has zero absorbance at this wavelength. Optimized method parameters for simultaneous equation and first order derivative spectroscopic methods are shown in Table 1.
Linearity
The calibration curves of ALS and AML were linear in the range of 25-300μg/ml and 5-100μg/ml respectively. Regression equation and R2 values are given in (Table 1).
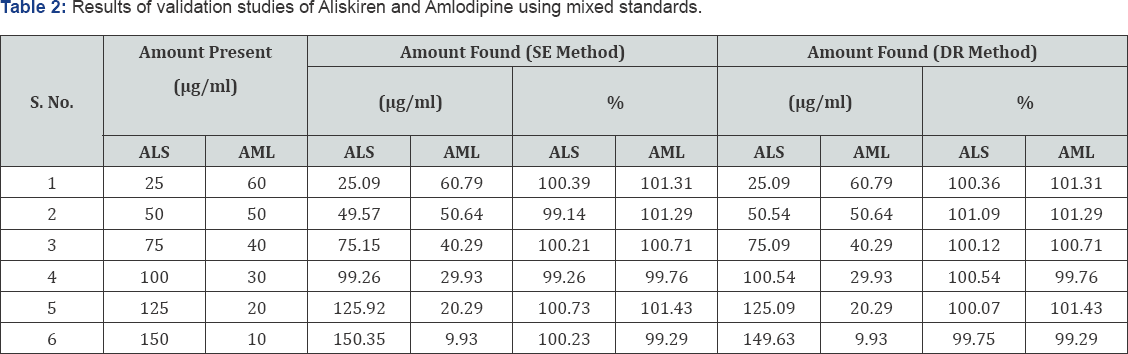
Standardization of the method by analysis of mixed standard solutions
The concentration of ALS and AML recovered from mixed standard solutions for both methods was within range and are given in Table 2.
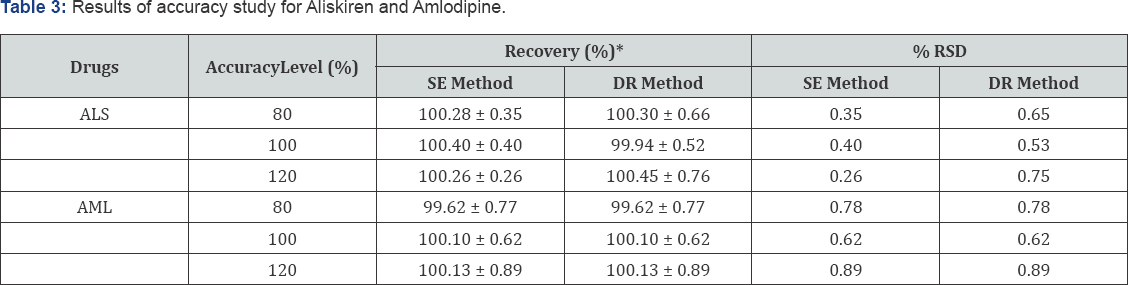
Accuracy
The percentage recoveries of drugs from sample were determined by standard addition of pure drugs at three known concentrations and recoveries were obtained at each level.The percent recoveries for ALS were found to be in the range of 100.26- 100.40% for SE method and 99.94-100.45 % for DR method. Percent recoveries for AML were found to be in the range of 99.62-100.13% for both methods. The results of accuracy studies are shown in Table 3.
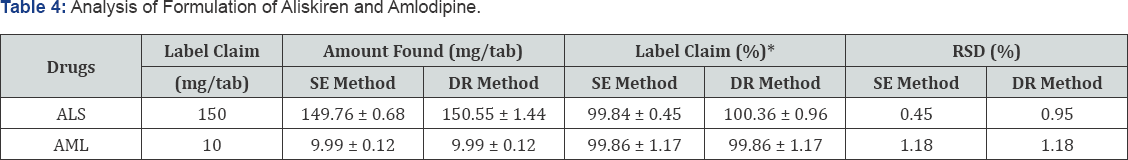
Application of the Method in Assay of Tablets
The proposed UV method was applied for the determination of ALS and AML in their combined pharmaceutical formulation and the results are shown in Table 4.
*Mean ± SD (n=3), SD (Standard deviation), %RSD (Percent relative standard deviation).
*Mean ± SD (n=5), SD (Standard deviation), %RSD (Percent relative standard deviation).
Conclusion
The proposed simultaneous equation method and first order derivative gives accurate and precise results for determination of aliskiren and amlodipine in marketed formulation (tablet) without prior separation and is easily applied for routine analysis. Method validation has been demonstrated by variety of tests like linearity, accuracy and validation through mixed standard. The proposed method can be successfully applied for determination of these drugs in commercial tablet formulation.
Acknowledgement
The authors express their sincere gratitude to Swapnroop Drugs and Pharmaceuticals, Aurangabad, Maharashtra, India for providing the pure drug samples of Aliskiren Hemifumarate and Amlodipine Besylate and are also thankful to colleagues and authorities of Department of Pharmacy, SRMSCET, Bareilly, U.P. who helped us in this work.
To know more about Journal of Organic and Medicinal Chemistry
Click here: https://juniperpublishers.com/omcij/index.php
To know more about Juniper Publishers
Click here: https://juniperpublishers.com/index.php




No comments:
Post a Comment