Civil Engineering Research Journal
The mechanical properties of sand-lime bricks produced by autoclaving under different conditions and incorporation of granulated slag have been investigated previously. In this study, the relationship is established between the structure, the phases formed and the strength. Based lime and granulated slag a new binder is developed. This is cured at saturated vapour pressures of 1.0 and 1.8MPa. The results showed a decrease in compressive strength due to the substitution. The microstructure analysis showed that reaction products consist mainly of 11Å tobermorite and xonotlite. Also, when increasing the autoclave temperature, it results in an increase in xonotlite relative to tobermorite. The X-ray diffractions of these phases are very low hardly visible, they are masked by the presence of quartz. Their intensities increase with the presence of slag.
Keywords: Sand lime bricks; Autoclaving; Granulated slag; Tobermorite; Xonotlite
Introduction
Calcium silicate brick is considered to be one of the advanced building materials in world and is made by hydrothermal reaction between sand or siliceous materials and lime. Despite many previous studies, several aspects of the reaction are still incompletely understood [1-3]. Under saturated vapour pressure and a temperature varying between 170 and 200 °C, fine-grained quartz (which is insoluble at ambient temperature) becomes chemically active and reacts with hydrated lime “Ca (OH)2”. This gives hydrated calcium silicate that is solid, resistant and insoluble in water [4]. These phases are often regrouped in the tobermorite appellation. The best known being the 14Å, 11Å or 9Å tobermorite; some are termed ‘normal’ while the others are called ‘anomalous’ with a Ca/Si ratio of 0.8-1.0 [5-7].
By virtue of its chemical composition, which is close to that of cement, the blast furnace slag can also be used instead of lime in the sand-lime materials. The rapid cooling of the slag results in a metastable glassy structure that favors its reaction with lime under specific conditions [8]. The blast furnace slag is known to possess latent hydraulic activity; i.e., it shows cementitious properties when in contact with water over a long period of time [9]. A chemical activation is necessary to start germination [10]. In addition, slag can also be reactive by thermal activation (in steam room and autoclave) [11].
Materials and Testing
Materials
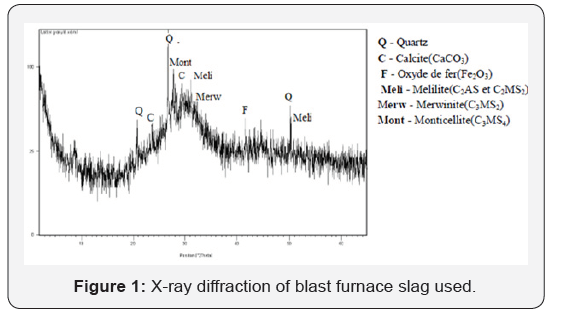
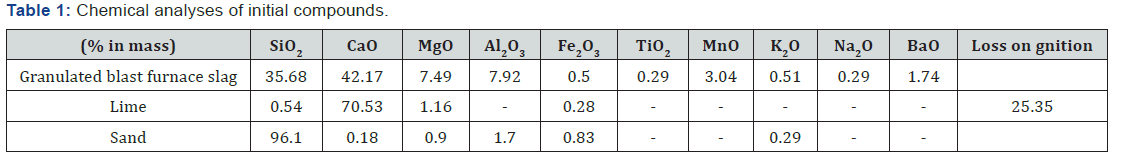
Granulated blast furnace slag (GBFS): The granulated blast furnace slag was provided by ArcelorMittal steel factory (situated in the east of Algeria). The chemical characteristic of slag is shown in Table 1. The slag was ground to a fineness of about 350 m2/kg (by Blain’s method) in a laboratory ball mill. Figure 1 Indicates the XRD patterns of the BFWS used. The crystal structure of GBFS is almost entirely amorphous since the XRD peaks are hardly identified. The angular band between 25° and 35° (2θ) could be attributed to a significant proportion of amorphous structure (glass). However, traces of mellilite, merwinite and probably monticellite at 2θ = 27.7°, can well be distinguished on more crystallized portions of granulated slag. It is likely that some slag agglomerates underwent partial crystallization during the cooling process. In addition to these minerals, traces of quartz, calcite and iron oxide are present [12-14].
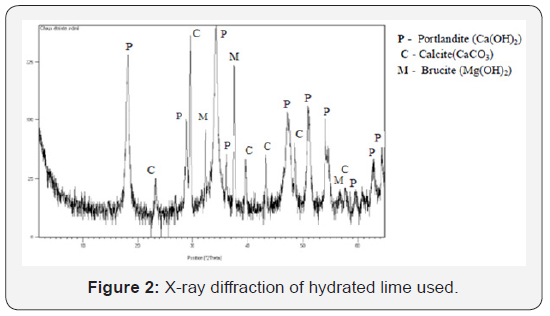
Hydrated lime: The quick lime is also collected from the ArcelorMittal Steel plant. Extinction and grinding were carried out at the Civil Engineering Research Laboratory, Annaba University. The chemical composition of the hydrated lime is given in Table 1. The X-ray diffraction diagram (Figure 2) shows a mineralogical composition of the hydrated lime primarily composed of portlandite Ca (OH)2. The presence of calcite is also noted. This is likely to be the result of carbonation of the portlandite. The brucite Mg (OH)2 (magnesium hydrate) is also identified: the limestone being slightly dolomitic.
Sand: The sand used in this work is collected from the siliceous dune sand of El-Kala area, eastern Algeria. It was ground to a specific area of 232.5m2/Kg and its chemical composition is given in Table 1.
Testing: To characterize the autoclaved sand-lime bricks, the cylindrical samples of 50mm in diameter and slenderness ratio of 2 were made by pressing in a hydraulic machine at a pressure of 20MPa. On the basis of previous study (Arabi 1988), a mixture of 20% hydrated lime and 80% ground sand was then humidified to 10% water. The granulated slag replaces hydrated lime partially. The samples were then put in a laboratory autoclave apparatus with 1.0 and 1.8MPa saturated vapour pressure corresponding to temperatures of 176° and 204 °C respectively. The cyclic treatment in autoclaving was 10 hours: 2 hours of progressively rising temperatures followed by 6 hours conservation at constant temperature then 2 hours of cooling by ventilation. The average of three tests results for each mixture was taken to characterize the compressive strength.
Results and Discussion
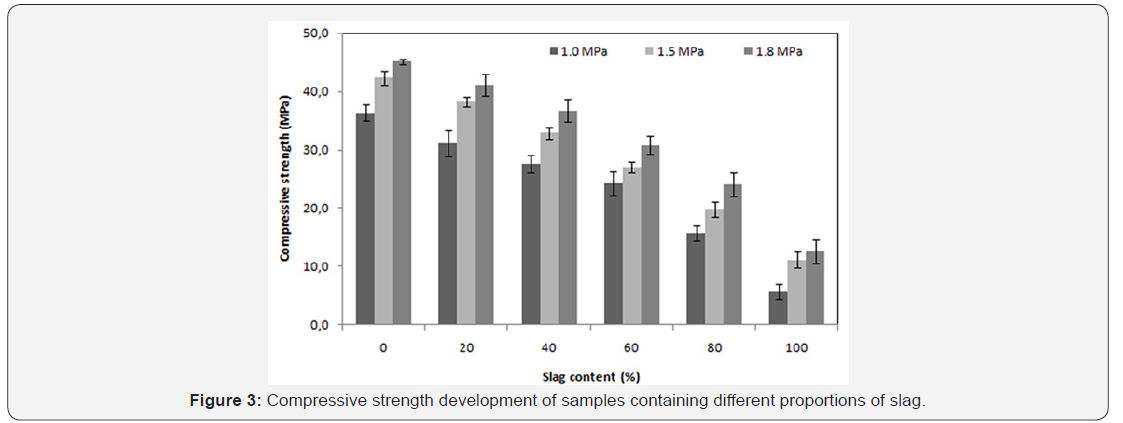
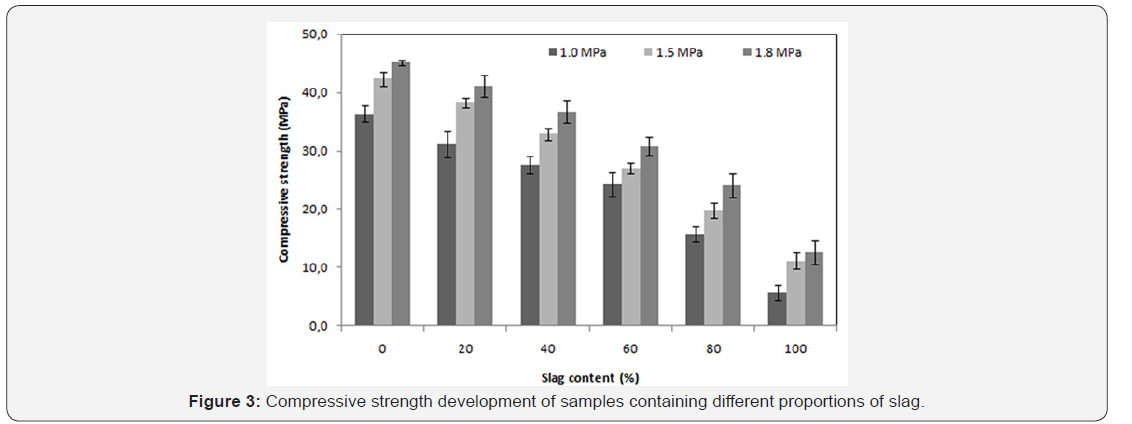
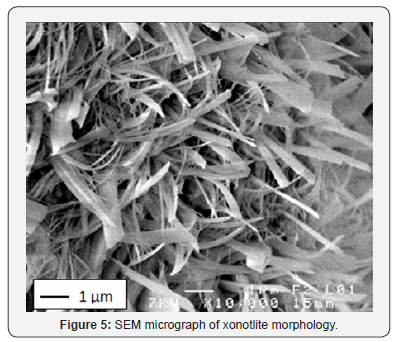
The mechanical strength is observed to improve significantly when the saturated vapor pressure, inside the autoclave, ranges from 1.0 to 1.5MPa. On the other hand, for the case of 1.5 to 1.8MPa saturated vapour pressure a slight improvement is noticed Figure 3. A significant increase of curing temperature can result in the phase breakdown followed by recrystallisation of other phases with weaker properties at microscopic scale (or at crystal scale). In the work reported by Black et al. [15]. concerning xonotlite and was not general to hydrothermally formed phases, an increasing synthesis temperature led to larger crystals, but the crystals also appeared to have split along their length. This case is also observed in this study: there is a conversion of the tobermorite to xonotlite. Figure 4 shows well-crystallized tobermorite in the form of dense and entangled platelets, whereas xonotlite is in the form of very fine interwoven needles Figure 5. However, at material scale the interleaving of xonotlite needles insures an improvement of mechanical strength.

The X-ray powder diffraction patterns Figure 6 shows a significant presence of portlandite in the sample without slag. The decrease of portlandite is induced by pozzolanic effect when the slag partially replaces hydrated lime, is also due to dilution. Portlandite disappears completely for mixtures to 60 and 100% granulated slag. The XRD patterns show at used temperatures and in the presence of slag, the poorly crystallized phases appear. The spectral line more visible is located at 7.8° 2θ; the XRD patterns from sample without slag do not show clearly.
Conclusion
It should be underlined the complexity of the CaO-SiO2-H2O system under hydrothermal reaction and saturated vapour pressure is widely discussed for many years. The presence of slag with almost a complete amorphous structure under such conditions, does not release new hydrate phases other than those known in similar conditions. The relatively high concentration of calcium ions is required for hydrate formation; the presence of lime is found to be essential as an activator for slag. The mechanical strengths are closely related to composition of mixtures. It was estimated that the increment of temperature has not an influence significant in improving resistance (in this study). However, the temperature acts much more on the morphology and crystalline degree of C-S-H. The usual forms of tobermorite and xonotlite are distinct and are not disturbed by the presence of slag. The investigation of phases by X-ray diffractograms is complicated by the presence of quartz (well crystallized and intensities that mask other phases).
To Know More About Civil Engineering Research Journal Please click on: https://juniperpublishers.com/cerj/classification.php




No comments:
Post a Comment