Juniper Online Journal Material Science
The objective of this work was to examine lignin fractionation from sugarcane bagasse using three organic solvents. Lignin derived from lignocellulosic sources such as sugarcane bagasse can be used as sources of low molecular weight aromatic compounds. These compounds can be used as chemical building blocks for the production of high value materials. In this work, organic solvent fractionation using methanol, ethanol and acetone were used to fractionate lignin from sugarcane bagasse. It was demonstrated that among these solvents the acetone and ethanol fractionated higher concentrations of lignin from bagasse. This indicated that these two solvents can be used to obtain lignin components from which low molecular weight aromatic components can be derived that have various applications.
Keywords: Lignin; Organic Solvent Fractionation; Low Molecular Weight Aromatic Compounds
Introduction
Lignin is a complex aromatic heteropolymer, which forms a component of lignocellulose contributing to the structural integrity of plants. Bugg, Ahmad et al. [1], Xu, Arancon et al. [2] Due to its aromatic composition, it is seen as a “green” source from which various aromatic building block chemicals can be derived. Xu, Hanna et al. [3] These chemicals are described as molecules with multiple functional groups making them readily convertible to a variety of high-value materials. Farmer and Mascal [4], Isikgor and Becer [5] This is because the functional groups can form new families of useful molecules with a variety of applications. Farmer and Mascal [4] One example of a building block compound derived from lignin is vanillin. This compound possesses phenolic among other functionalities and is currently being investigated in polymer chemistry for production of polyesters among other types of polymers. Constant, Robitzer et al. [6], Sainsbury, Hardiman et al. [7], Fache, Boutevin et al. [8].
Currently the main source of lignin is from black liquor produced as a by-product from the pulp and paper industry. Park, Kim et al. [9] However, due to the heterogeneity of lignin and it low solubility the use of lignin as a source of aromatic compounds has been limited. Alternatively, methods using various organic solvents can fractionate components of lignin. This leads to more homogenous lignin fractions, from which consistent aromatic compounds can be derived. Zhao, Cheng et al. [10], Fiţigău, Peter et al. [11], Ragauskas, Beckham et al. [12], Sadeghifar, Wells et al. [13], Park, Kim et al. 2018 [9]. In this examination, organic solvent fractionation, which is a low energy process, was used to target the lignin component of bagasse. This process causes the disruption and removal of low molecular weight components of the lignin. Zhao, Cheng et al. [10], Fiţigău, Peter et al. [11] Organic solvents include alcohols such as methanol and ethanol, ketones such as acetone and glycols such as glycerol and ethylene glycol. Among these, commonly used organic solvents are methanol, ethanol and acetone. Fiţigău, Peter et al. [11], Sadeghifar, Wells et al. [13] These solvents work by cleaving ether and ester linkages in the lignin. Sugarcane bagasse is a lignocellulosic material which has high lignin content (11-25%, dry weight basis) and can be used as a renewable source of lignin. Chandel, da Silva et al. [14] Therefore the aim of this work was to examine the application of organic solvent fractionation using methanol, ethanol and acetone to derive low molecular weight lignin components.
Materials and Methods
All chemicals used in this work were obtained from Sigma Aldrich, St. Louis MO, USA unless otherwise stated.
The organic solvents consisted of two alcohols: methanol and ethanol and one ketone: acetone. Solvent water solutions were prepared at concentrations of: 10%, 30%, 50%, 70%, 90% and 100% (v/v). Zhao, Cheng et al. [10], Fiţigău, Peter et al. [11] Bagasse (1 g), was mixed with 50 mL of solvent solution in 125 mL flasks, which were set at room temperature for 24 hours without stirring. After this time, the bagasse residues were separated from the filtrate by gravity filtration. The concentration of lignin in this filtrate was estimated spectrophotometrically from a standard curve constructed using various concentrations (0 - 10 mg/ mL) of Kraft lignin in 1 M NaOH. Park, Kim et al. [9] The absorbance of lignin was determined by a wavelength scan from 190 - 800 nm, using single beam UV - Vis spectrophotometry (Agilent 8453 UV-Vis spectrophotometer, Shimadzu UV-1280). Fiţigău, Peter et al. [11], Sadeghifar, Wells et al. [13], Park, Kim et al. [9]. The liquor obtained from the fractionation process was analyzed spectrophotometrically to confirm the presence of lignin. This was done by evaporating the solvent under reduced pressure followed by lyophilization. The dried brownblack residue (5 mg) was dissolved in 1 M NaOH using a 10- mL volumetric flask. Fiţigău, Peter et al. [11], Wildschut, Smit et al. [15], Jääskeläinen, Liitiä et al. [16] Two ten-fold dilutions were made to produce a straw-colored solution for analysis. Additionally, Kraft lignin was utilized for comparison purposes. The absorbance of the solutions was found by wavelength scan from 190 to 1100 nm using UV- Vis spectrophotometry (Agilent 8453 UV-Vis spectrophotometer). Dominant peaks between 250 - 350 nm were related to lignin components extracted from the bagasse. Sun, Sun et al. [17], Tolbert, Akinosho et al. [18]. The program GraphPad Prism 7.01 was used for the statistical analysis and to construct the graphs displayed. In cases where the error bars are shorter than the height of the symbol, Prism does not draw those error bars.
Results and Discussion
The presence of lignin components in the liquor derived from fractionation was confirmed by two dominant peaks at 291 and 331 nm. These results were compared to those collected for Kraft lignin alkali, which demonstrated dominant peaks at 219 and 288 nm. de la Torre, Moral et al. [19], Fiţigău, Peter et al. [11] This is the typical range at which lignin absorbs, demonstrating that the fractionation process removed components of lignin from the bagasse. Fiţigău, Peter et al. [11], Jääskeläinen, Liitiä et al. [16] The observed peak shift between solvent fractionated lignin and Kraft lignin is caused by the difference in lignin composition. Kraft lignin is derived from the Kraft pulping processes, which depolymerizes lignin into small aromatic derivatives using hot water, NaOH and Na2(SO3). Zhao, Cheng et al. [10], Espinoza- Acosta, Torres-Chávez et al. [20] These smaller fragments absorb UV light at a lower wavelength. This is relative to larger, more intact fragments of lignin, derived from the organic solvent fractionation process. These fragments tend to absorb UV light at a higher wavelength. Xu, Hanna et al. [3], Park, Kim et al. [9]. Further, the data collected from organic solvent fractionation of bagasse by acetone, ethanol and methanol, are displayed as mean ± SEM in Figure 1 and Table 1. Statistical analysis was performed to compare the means of lignin concentration fractionated from bagasse. For each of the three solvents, the highest mean lignin concentrations were compared using one-way ANOVA (alpha = 0.05).
The results presented in Figure 1 show that lignin is fractionated from bagasse in the order of acetone > ethanol > methanol between 10 - 70% solvent concentration. For acetone and ethanol, the highest concentrations of lignin were fractionated at solvent concentrations of 50% (Figure 1) and (Table 1). At concentrations above 90% (v/v), fractionating ability decreased. For methanol, the highest concentration of lignin was fractionated at 70% (v/v). It was also observed that after 50 - 70% solvent concentration, the lignin concentration generally decreased. This is related to the molecular weight of the lignin fragments. At solvent concentrations of 50 - 70%, maximal quantities of highly soluble lower molecular components are fractionated by the solvents examined. These components are high in free hydroxyl and carboxylic regions which significantly increase solubility. However, at higher solvent concentrations, higher molecular weight and less soluble lignin components are fractionated. Jääskeläinen, Liitiä et al. [16], Park, Kim et al. [9] This occurrence has been previously observed in fractionation of Kraft lignin with ethanol and also fractionation of several technical lignin’s with acetone Fiţigău, Peter et al. [11].
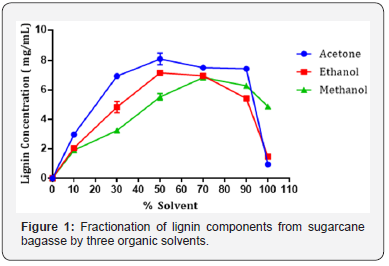
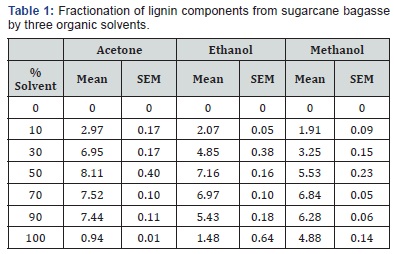
One-way ANOVA for the three solvents at 50% (v/v) showed that the effect of the solvent was significant (F (2, 6) = 21.28, p < 0.05). Post hoc analysis using Tukey HSD multiple comparison testing indicated that the concentrations of lignin fractionated by acetone at 50% v/v (Tukey, p < 0.05) was significantly higher than for methanol. Concentrations of lignin fractionated by ethanol at 50% v/v (Tukey, p < 0.05) were also significantly higher than for methanol. Conversely, the concentration of lignin fractionated by ethanol and acetone, both at 50% v/v were not significantly different (Tukey, p > 0.05). From this analysis, it was determined that either acetone or ethanol at 50% (v/v) could be selected a better solvent for the organic solvent fractionation of lignin components from bagasse Abdelaziz, Brink et al. [21], Fache, Boutevin et al. [8].
Conclusion
These results indicate that organic solvent fractionation can be applied directly to bagasse lignocellulosic material to obtain lignin components. Further, acetone and ethanol demonstrated similar ability to fractionate lignin from bagasse. The lignin components obtained can then be used as sources of low molecular weight aromatic compounds including vanillin, which can be used as chemical building blocks.
To Know More About Juniper Online Journal Material Sciences Please click on:https://juniperpublishers.com/jojms/




Wow, that is quite informative. I like this article very much. The content was good. If any of the engineering students are looking for a projects for ece final year students, I found this site and they are providing the best service to the engineering students regarding the projects power electronics based projects
ReplyDelete