Authored
by Mostafa M Sira
Abstract
Background: Biliary Atresia (BA) is the most common cause of chronic cholestasis in infants It is a destructive inflammatory obliterative cholangiopathy that affects varying lengths of both intrahepatic and extrahepatic bile ducts. Even after a successful surgery, scARGHing of the liver can continue, resulting in cirrhosis and its complications.
Aim: The aim of this study is to evaluate different serological markers derived from routine investigations in the prediction of liver fibrosis in infants with BA.
Methods: This retrospective study included a total of 147 infants with proved diagnosis of BA. We employed six noninvasive scores (FIB-4, FibroQ, King’s score, APRI, GUCI and AAR). Liver fibrosis was classified into 5 grades. For further descriptive purpose, we arbitrarily divided fibrosis grades into early (F1, F2 and F3) and advanced (F4 and F5) fibrosis.
Results: FIB-4, FibroQ and King’s score correlated significantly with fibrosis grade (P values were 0.007 and 0.015 respectively) while there was no significant correlation with other studied scores (P value >0.05). FIB-4, FibroQ and King’s score were significantly higher in patients with advanced fibrosis compared to early fibrosis and at cutoff values of 0.0098, 0.0085 and 0.115 respectively they were able to discriminate those with advanced fibrosis with acceptable sensitivity (61.9%-64.3%) and specificity (60.0%-62.9%).
Conclusion: Conclusion: FIB-4, FibroQ and King’s score, but not APRI, GUCI and AAR, correlated significantly with fibrosis and could predict those with advanced fibrosis with relatively acceptable performance. These markers may be of help in predicting advanced fibrosis and in long term follow up of infants with BA and reduce the need for repeated liver biopsy.
Keywords: AAR; APRI; Biliary atresia; FIB-4; FibroQ; GUCI; King’s score; Liver fibrosis; Noninvasive; Serological markers
Abbreviations: BA: Biliary Atresia; AAR: AST/ALT Ratio; ALP: Alkaline Phosphatase; ALT: Alanine Transaminase; APRI: AST-To-Platelet Ratio Index; AST: Aspartate Transaminase; AUROC: Area Under ROC; FIB-4: Fibrosis-4; FibroQ: Fibro-Quotient; GGT: Gammaglutamyl Transpeptidase; GUCI: Göteborg University Cirrhosis Index; INR: International Normalized Ratio; NPV: Negative Predictive Value; PPV: Positive Predictive Value; ROC: Receiver Operating Characterstic
Introduction
Biliary Atresia (BA) is the most common cause of chronic cholestasis in infants and the most frequent cause for surgery in cholestatic jaundice in this age group. It is a destructive inflammatory obliterative cholangiopathy that affects varying lengths of both intrahepatic and extrahepatic bile ducts [1]. If not treated, BA leads to biliary cirrhosis, hepatic failure and death within the first two years of life [2,3].
The etiology of BA has been a subject of intense investigation. However, the precise etiology remains largely unknown [4]. The initial event may be a viral infection, which targets the biliary epithelium [5]. This is followed by activation of immune cells and release of proinflammatory cytokines that perpetuates the injury and causes biliary destruction, which is followed by collagen deposition to produce the atresia phenotype [6]. Some studies suggested the involvement of biliary morphogenesis genes [7,8] or very recently discovered biliary toxin; biliatrisone [9,10].
The principal treatment of BA is based on surgical reconstruction of bile flow by Kasai portoenterostomy. However, such interventions can be insufficient to prevent further hepatic injury. Even after a successful surgery, scARGHing of the liver can continue, resulting in cirrhosis over the years. This is probably due to the ongoing inflammatory process [11].
Complications of progressive fibrosis and cirrhosis such as esophageal varices may endanger the patient’s life and necessitates urgent intervention [11]. Furthermore, the success of Kasai portoenterostomy is largely dependent on the absence of advanced fibrosis or cirrhosis [12]. For that, noninvasive prediction of liver fibrosis in such patients, avoiding the risks of repeated liver biopsy [13,14] and its limitations including sampling error, and inter- and intra-observer variability in interpretation [15], would be of value during monitoring and follow up of this devastating disease [16]. The aim of the current study was to evaluate different serological markers derived from routine laboratory investigations in the prediction of liver fibrosis in infants with BA.
Patients and Methods
Study population and data collection
This retrospective study included 147 infants with surgically proved BA attending the Department of Pediatric Hepatology, Gastroenterology and Nutrition in the period between year 2010 and 2015. Preoperative demographic (age and sex), laboratory data including total and direct bilirubin, transaminases (alanine transaminase; ALT and aspartate transaminase; AST), biliary enzymes (gammaglutamyl transpeptidase; GGT and alkaline phosphatase; ALP), total proteins, serum albumin, international normalized ratio (INR) and platelets count were collected. Hepatic histopathological features in the form of portal fibrosis, were also revised. Due to the retrospective nature of the study, an informed consent was not needed. The study was approved by the Research Ethics Committee of the National Liver Institute, Menofiya University, Egypt.
Laboratory investigations
Fifteen milliliters venous blood samples were taken by sterile venipuncture, without frothing and after minimal venous stasis using disposable syringes. The blood samples were distributed as follows: 5 ml of venous blood were delivered in a vacutainer plain test tube. Blood was left for a sufficient time to clot; serum was then separated after centrifugation at 3000 rpm/min for 10 min for liver function tests. Five milliliters of venous blood were delivered in a vacutainer plastic tube containing EDTA for complete blood count (CBC). Five milliliters of venous blood were delivered in a vacutainer plastic tube containing Sodium Citrate for INR. CBC was performed on Sysmex KX-21 (Wakinohamakaigandori, Kobe, Hyogo, Japan). Liver function tests [ALT, AST,albumin, total protein, total bilirubin, direct bilirubin, ALP and GGT] were conducted using Integra 400 autoanalyzer (Roche- Diagnostics, Mannheim, Germany). Prothrombin time and INR were conducted using Sysmex CA 1500 coagulometer
infection received peg-interferon and ribavirin treatment for 48 weeks, out of nine patients showed Resistance to the treatment. Blood sampling were made on at start and end of the treatment. Based on the therapeutic response to antiviral treatment, those 18 patients could divide into two groups: Treated (Responder, R) 9 patients, and Resistant (Non-responder, NR) 9 patients.
Liver biopsy
Ultrasonography-guided liver biopsy was done for all patients using a tru-cut needle. Biopsy specimens were fixed in formalin and embedded in paraffin. Five-micron thick sections were cut and stained with Hematoxylin-Eosin, Mason-Trichrome, Orcein and Perls’ stains for routine histopathological evaluation. Portal fibrosis was assessed using a semi-quantitative histopathological score as described by Russo et al. [17].
Calculation of the selected non-invasive serological scores
The employed scores was calculated as follows; AST-toplatelet ratio index (APRI) was calculated according to the formula; APRI = AST / upper limit of normal x 100 / platelet count (109/L) [18]; Fibrosis-4 (FIB-4) = Age (years) x AST / platelet count (109/L) x (ALT)1/2 [19]; Fibro-quotient (FibroQ) index using this formula 10 × (age in years × AST × INR)/(ALT × platelet count) [20]; King’s score using this formula Age (years) x AST (IU/L) x INR/platelet count (109/L) [21]; AST/ALT ratio (AAR) [22]; Göteborg University Cirrhosis Index (GUCI) using the formula (Normalized ASTxINRx100)/platelet count (109/L) [23].
Statistical Analysis
This retrospective study included 147 infants with surgically proved BA attending the Department of Pediatric Hepatology, Gastroenterology and Nutrition in the period between year 2010 and 2015. Preoperative demographic (age and sex), laboratory data including total and direct bilirubin, transaminases (alanine transaminase; ALT and aspartate transaminase; AST), biliary enzymes (gammaglutamyl transpeptidase; GGT and alkaline phosphatase; ALP), total proteins, serum albumin, international normalized ratio (INR) and platelets count were collected. Hepatic histopathological features in the form of portal fibrosis, were also revised. Due to the retrospective nature of the study, an informed consent was not needed. The study was approved by the Research Ethics Committee of the National Liver Institute, Menofiya University, Egypt.
Results
Study population’s characteristics
The current study included 147 infants with BA. Their mean age was 76 ± 41 days and 55% were females. Other baseline laboratory parameters and histopathological fibrosis grades were as presented in Table 1.
Distribution of serological scores according to fibrosis grades
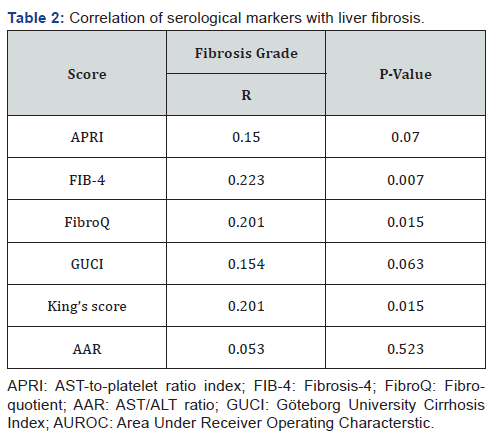
The selected scores were compared according the individual fibrosis grades. In all the six scores, the values were at its lowest in F1 and was highest in F5 except for FibroQ and AAR, the values were lower than that of F4, yet, there was no significant statistical difference among the different grades of fibrosis (Figure 1). On the other hand, correlation analysis revealed a significant positive correlation of FIB-4, FibroQ and King’s scores with fibrosis grades (P values were 0.007 and 0.015 respectively) while there was no significant correlation with the other studied scores (P value >0.05) as shown in Table 2.
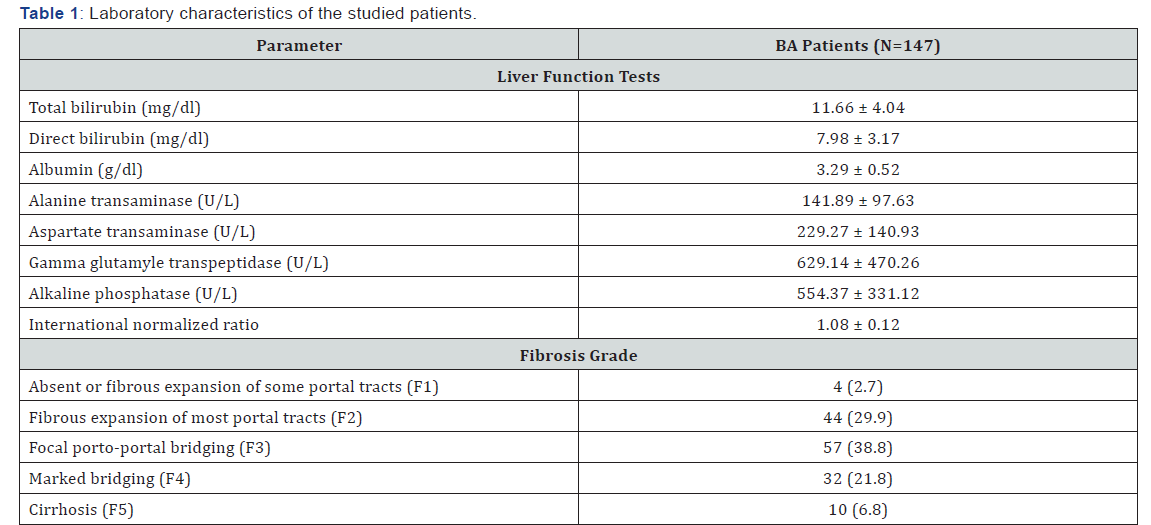
APRI: AST-to-platelet ratio index; FIB-4: Fibrosis-4; FibroQ: Fibro-quotient; AAR: AST/ALT ratio; GUCI: Göteborg University Cirrhosis Index.
Comparison between early and advanced fibrosis
For descriptive purpose, we arbitrarily divided fibrosis grades into early (F1, F2 and F3) and advanced (F4 and F5) fibrosis. Again, FIB-4, FibroQ and King’s scores showed a significantly higher values in those with advanced fibrosis (P values were 0.007, 0.017 and 0.009 respectively) while there was no significant difference using the other studied scores (P value >0.05) as shown in Table 3.

Performance of FIB-4, FibroQ and King’s scores in discriminating advanced fibrosis
The three scores (a cutoff value of 0.0098 for FIB-4; 0.0085 for FibroQ and 0.115 for King’s score) showed nearly a comparable performance in discriminating advanced fibrosis (Table 4).

Discussion
The prognosis of chronic cholestatic diseases depends, in part, on the extent of liver fibrosis [24,25], while it markedly influences the outcome of Kasai protoenterostomy in infants with BA [12]. In addition, it identifies those in need of liver transplantation whether in those who performed a previous Kasai operation or not [26,27] For that follow up of fibrosis progression is of utmost importance. Liver biopsy, being the gold standard in assessment of liver fibrosis, is not largely accepted when repeated, especially in the pediatric population. For that , the use of noninvasive predictor of liver fibrosis is needed [28,29].
Several noninvasive markers and scores have been applied satisfactorily in hepatitis C virus [18] and non-alcoholic fatty liver diseases [30], while studies on its use in BA are very limited. APRI score has been used in predicting liver fibrosis in BA. Yet, the results are contradictory. Kim et al. [31] reported that APRI significantly discriminated F3 and F4 Metavir score in infants with BA. AUROC for F≥3 and F=4 were 0.92 and 0.91, respectively. Distinct optimal cutoff values of APRI for F≥3 and F=4 were obtained (1.01 and 1.41, respectively). In addition, Grieve et al. [16] using a cutoff value of 1.22 [AUC 0.83] showed a sensitivity of 75% and a specificity of 84% for macroscopic cirrhosis. Native liver survival was significantly different but improved only for those with the lowest APRI quartile (P=0.009). Similar results were also reported by Yang et al. [32].
On the other hand, Lind et al. [33] found that APRI did not significantly differ in various fibrosis Metavir scores (P = 0.89) and was not correlated with transplant-free survival (r=0.08; P=0.67) in infants with BA. Our results are in agreement with that of Lind et al where APRI value neither differ significantly with different Russo fibrosis grades (P = 0.445) nor correlated with fibrosis (r=0.15; P = 0.07). Nonetheless, APRI values increased successively as fibrosis increases with its lowest in F1 and highest in F5.
Other scores have been used in predicting fibrosis in HCV, all of which are dependent on the routine laboratory tests regularly performed in these patients. Leung et al. [34] found that APRI performed better than FIB-4 in predicting fibrosis studied in children with cystic fibrosis liver disease. In the current study, contrary to APRI, FIB-4 was significantly correlated with fibrosis in BA (P = 0.007) and was significantly higher in those with advanced fibrosis (Russo F4 and F5; P=0.007). With AUROC of 0.644, FIB-4 could predict advanced fibrosis with 61.9% sensitivity and 61.9% specificity. On the other hand, Chen et al. [35] reported that FIB-4 failed to correlate with fibrosis stage. This may be due to the small number of patients in Chen’s study (n = 24) compared to our study (n = 147).
GUCI and AAR were able to predict fibrosis in HCV and hepatocellular carcinoma in addition to predicting response to antiviral therapy [36-38]. In our study, both scores were not correlated with liver fibrosis (P = 0.063 and 0.523 for GUCI and AAR respectively) and could not discriminate advanced from early fibrosis. Unfortunately, there are no reported studies for their use in BA.
On the other hand, FibroQ and King’s score showed a significant positive correlation with fibrosis grade (P = 0.015 for both) and at a cutoff value of 0.085 and 0.115 respectively, both could discriminate advanced fibrosis from early fibrosis with comparable sensitivity (64.3% for both) and specificity (60.0% and 62.9% respectively). King’s score has been used in assessing fibrosis in chronic hepatitis B [39] and hepatitis C [21] but no reports about its use in predicting fibrosis in BA. Combining the three scores (FIB-4, FibroQ and King’s score) did not improve the performance compared to the performance of each score individually. Although statistically significant, the performance of these scores was found to be better in adult studies with chronic hepatitis C. This may be due to the fibrogenic nature of BA and the relatively high platelet counts even in cases with advanced fibrosis [40] which may influence the performance of platelet count-based scores.
In conclusion, FIB-4, FibroQ and King’s, but not APRI, GUCI or AAR, correlated significantly with fibrosis grade in infants with BA. These noninvasive serological markers, which are derived from simple routine laboratory tests, may be of help in predicting advanced fibrosis and in long term follow up of infants with BA, and minimize the need for repeated follow up liver biopsies.




No comments:
Post a Comment