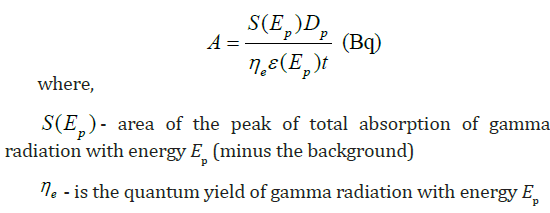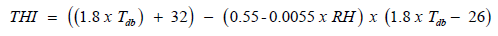Heat stress (HS) has detrimental effects on lactating
cattle especially when they are in a negative energy balance. Feed
additives have been shown to mitigate the effects of HS by improving
metabolic and immune function. The objective of this study was to
evaluate the effect of feeding a dietary supplement (Land O’Lakes Inc.,
Arden Hills, MN) on the HS response in multi-parturient dairy cows in
mid lactation. Two pens of cows at a commercial dairy were fed either
control (CON) or additive (YB) at approximately 56.5 kg/pen per d for
two weeks prior to arrival. Study cows (n=12) were balanced in days in
milk (DIM), milk production, and parity (111.91±4.85 d, 33.67±0.96 kg/d,
and 2.25±0.18). On arrival, the cows (6 YB and 6 CON) were randomly
selected and housed in environmentally controlled chambers for 18 d and
fed appropriate diet. Cows were subjected to 7 d of thermoneutral (TN)
conditions, 7 d of HS, and 4 d of recovery (REC) under TN conditions.
Dry matter intake (DMI), milk production from AM and PM milkings, and
milk composition were measured daily. Rectal temperature (RT) and
respiration rate (RR) were measured at peak temperature daily. Blood
samples were collected once daily at 0900 h following catheterization on
d 5 of TN to d 4 of REC. Serum samples were analyzed for glucose,
insulin, blood urea nitrogen (BUN), β-hydroxybutyrate (BHB), and
non-esterified fatty acids (NEFA). Results were analyzed using repeated
measures in the PROC MIXED of SAS. HS increased RT (P<0.0001), RR
(P<0.0001), BUN (P<0.0001), BHB (P=0.0009), insulin (P=0.036),
cortisol (P<0.0001), neutrophil (P=0.0272), and water intake
(P<0.0001). HS decreased lymphocyte (P<0.0001), DMI (P<0.0001),
and 4% fat corrected milk (FCM, P=0.02). YB increased the feed
efficiency ratio (P=0.03). YB had no effect on blood parameters. There
was a treatment interaction with cows fed YB having higher feed
efficiency (P=0.05) during peak thermal loads than CON. Results of this
study suggest that HS exposure had performance and metabolic impacts in
mid lactation cows. Supplementation with YB alleviated some of the
performance effects associated with HS.
Keywords: β-hydroxybutyrate; Altering cortisol; Rumen acidosis; Ketosis; Lameness; Mastitis; Metritis
Abbreviations: HS: Heat
stress; DIM: Days In Milk; TN: Thermoneutral; REC: Recovery; DMI: Dry
Matter Intake; RT: Rectal Temperature; RR: Respiration Rate; BUN: Blood
Urea Nitrogen; BHB: β-Hydroxybutyrate; NEFA: Non-Esterified Fatty Acids;
THI: Temperature-Humidity Index
HS is a costly factor to the dairy industry [1] as it
changes the livestock production system due to increasing climate
variability [2]. Physiological responses occur when the
temperature-humidity index (THI) exceeds 68 or when the ambient
temperature exceeds 32.2˚C [3]. Measurable factors that are negatively
affected by heat stress are milk production, dry matter intake, growth,
and health disorders [4]. It has been shown that milk yield is partially
affected by HS through independent mechanisms of reduced nutrient
intake [5].
Increasing the energy density of the diet from
carbohydrates sources to fatty acid sources are common methods for
feeding
heat stressed animals but increase the chances of rumen acidosis per
Kadzere et al. [6]. Using supplemental dietary additives have been shown
to stabilize the rumen and increase post-rumen nutrient flow [7] in
non-stressed cows; however, Shwartz et al. [8] reported no effect of a
yeast culture on mitigating the effects of heat stress on lactating
cows. The feed additive used in this study was a proprietary phytogenic
and specialty yeast blend (Land O’Lakes Inc., Arden Hills, MN). Proven
feed additives have shown increased immune function in lactating dairy
cows [9] by altering cortisol responses in supplemented cows [10].
We hypothesized that supplementing with the YB would
increase energy availability and thus reduce the negative effect of the
heat load on production measures. The study objectives were
to evaluate the effects of the dietary YB on body, production, and
metabolic parameters in heat stressed lactating cows.
This study was conducted at the Agricultural Research
Complex (ARC) at the University of Arizona (Tucson, AZ). The
protocol was reviewed and approved by the University of Arizona
Institutional Animal Care and Use Committee. It occurred in two
phases: on-farm and on-site.
The on-farm phase consisted of feeding one 500 cow pen and
monitoring a control pen of the same number of cows at a dairy
in Stanfield, AZ. Treatment cows were given the feed additive in
the TMR at 56.5 kg/pen and received it for 2 weeks. Previous
research has shown that there may be a cell-mediated response
in the rumen and that requires time to see a response during
heat stress [10]. After the 2-week period, 6 treatment cows and 6
control cows were randomly selected and shipped to the ARC for
the on-site phase with no health issues (rumen acidosis, ketosis,
lameness, mastitis, metritis).
Upon arrival, twelve multiparous Holstein cows producing
33.67 ± 0.96 kg of milk/d, lactation (2.25 ± 0.18), and stage of
lactation (111.92 ± 4.85 DIM) were weighed and randomly assigned
to individual tie stalls in one of two environmental chambers. Both
chambers housed 6 cows (3 control and 3 treatment). Chambers
operated on the same environment throughout the study. There
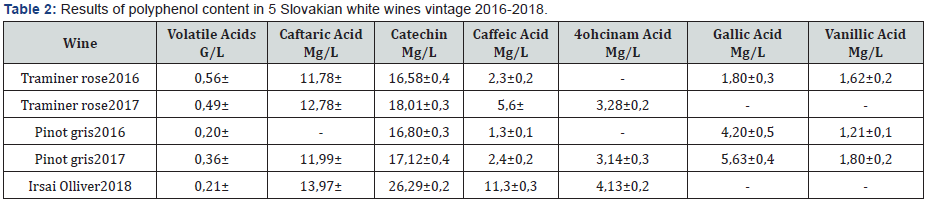
were 4 periods: acclimation (4 days), thermal neutral (TN, 7
days), heat stress (HS,7 days), and recovery period (4 days)
(Figure 1). The acclimation period and TN period were set on
the same environmental cycle. The TN environment was set at a
minimum temperature of 21.16 ̊C, a maximum of 21.48 ̊C, and an
average of 21.39 ̊C. The relative humidity (RH) had a minimum
of 28.32 %, a maximum of 36.73 %, and an average of 32.30 %.
The temperature-humidity index (THI) had a minimum of 65.54,
a maximum of 66.16, and an average of 65.84 (Figure 2). The
HS period had a minimum temperature of 25.40 oC, a maximum
of 34.89 oC, and an average of 30.09 oC. The RH had a minimum
of 23.40 %, a maximum of 36.48 %, and an average of 30.88 %.
The THI had a minimum of 70.68, a maximum of 80.70, and an
average of 75.46 (Figure 2). The Recovery period had a minimum
temperature of 21.11 oC, a maximum of 21.43 oC, and an average of
21.38 oC. The RH had a minimum of 40.46 %, a maximum of 50.93
%, and an average of 45.93 %. The THI had a minimum of 66.39, a
maximum of 66.99, and an average of 66.78 (Figure 2). The THI of
68 identifies as the threshold for HS in lactating dairy cows [11].
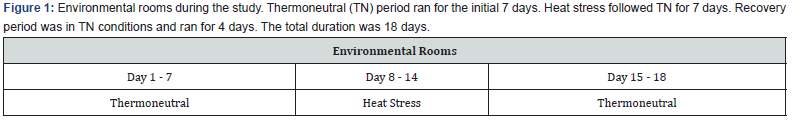
THI values were calculated using the average temperature and
relative humidity obtained from the data logger. THI was defined
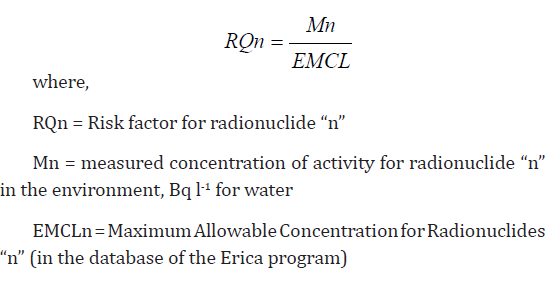
by the formula [12]:
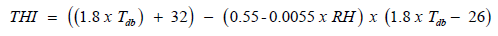
Where: db T = dry bulb temperature (̊oC), RH = relative humidity
percentage.
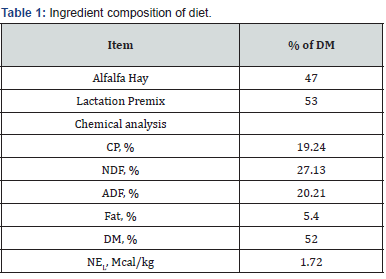
The diet was an alfalfa based TMR that was balanced to be
consistent with the green chop based TMR on the commercial
dairy. Grab samples were collected once every 3 days when a new
batch of feed was mixed. Alfalfa was added to the TMR pre-mix
and was stored at 4 oC. Samples were analyzed by Dairy Nutrition
Services INC (Chandler, AZ) by wet chemistry
Feeding and milking were done twice daily at 0530 and 1730
h. The CON cows were fed the base TMR. The YB cows were fed the
TMR plus 113.4 g of YB mixed. Orts were removed and weighed in
the morning prior to feeding. The feed efficiency was determined
by using 4% fat corrected milk (FCM) per kilogram of DM consumed
for each cow in each treatment group. Water consumption was
recorded daily from the water meters after the AM feeding. Milk
weights were recorded daily and a daily milk sample was taken
from the AM milk. Milk samples were individually refrigerated
with a preservative (Bronopol tablet, D&F CON Systems, San
Ramon, CA) at 4 oC. Samples were analyzed for fat, lactose, protein,
somatic cell count (SCC), and solids-not-fats (SNF) by Arizona
DHIA (Tempe, AZ). Body weights were recorded twice during the
study: once during HS and at the beginning of REC.
Physiological measurements such as respiration rate and
rectal temperature were recorded for each cow once daily at
1400 h. Respiration rate was recorded as breaths per minute.
It was calculated by counting the flank movements for 15s and
multiplying by 4. Rectal temperature was measured using a
Cooper TM99A thermometer (Cooper-Atkins, Middlefield, CT).
Cows that exceeded 40.5oC were removed from the chamber
and were cooled with water until a rectal temperature of 38.3oC.
Vaginal temperatures were recorded with HOBO® U12 stainless
steel temperature data loggers (Onset Computer Corp., Bourne,
MA) in 5 minutes increments. Blank controlled internal drug
releasing devices (Eazi-BreedTM CIDR®; Zoetis, Parsippany-Troy
Hills, NJ) were used to hold the HOBO in the vagina. The cows were
fit with this apparatus on d 1 for the duration of the study. CIDR
placement was monitored and were refitted if they were expelled
from the vagina.
Blood was collected via indwelling jugular catheters that were
surgically inserted into cows on day 4 and 5. Blood was collected at
0900 h from day 5 to 18. Catheters were flushed before sampling
and once at 1500 h with heparinized saline (100 USP/mL). The
first 6 mL of fluid was discarded to eliminate heparin. Two 12 mL
syringes with a 22-gauge needle were used to draw blood and
were transferred to BD Vacutainer tubes (BD, Franklin Lakes, NJ):
sodium heparin and blank. Samples were chilled for one hour at
4 oC. Serum and plasma were collected after centrifuging samples
at 1,500 x g for 15 min at 4 oC and stored at -80 oC until analysis.
Additional blood samples for white blood cell differentials were
collected in BD Vacutainer tubes with EDTA. Differentials were run
by Antech Diagnostics (Phoenix, AZ).
Serum β-hydroxybutyrate (BHB) was determined by a
colorimetric kit (β-hydroxybutyrate (Ketone Body); Cayman
Chemical, Ann Arbor,MI). Serum blood urea nitrogen (BUN) was
determined using a colorimetric kit (DetectX Urea Nitrogen; Arbor
Assays, Ann Arbor, MI). Serum glucose was measured using a
colorimetric assay (Glucose Oxidase; Pointe Scientific Inc., Canton,
MI). Serum non-esterified fatty acid (NEFA) was determined
enzymatically through a commercial kit (Wako NEFA-HR(2); Wako
Chemicals USA, Richmond, VA). Serum insulin was determined
using an enzyme linked immunosorbent assay (ELISA) kit (Bovine
Insulin, ELISA kit; ALPCO, Salem, NH).
Plasma cortisol was assessed using an enzyme immunoassay
(EIA) kit (Cortisol Parameter Assay; R&D Systems Inc., Minneapolis,
MN) with an intra- and interassay CV <5 %.
Physiological and blood data were analyzed using a PROC
MIXED model as a 2 x 2 replicated factorial design with the
LSMEANS and PDIFF options within each environmental period
as the REPEATED option with day within period as the repeated
measure, and effect of the cow was nested within treatment,
using a covariant structure. Rectal and vaginal temperatures were
averaged per hour by day of trial. Environment (ENV), treatment
(TRT), ENV x TRT, day(ENV), and treatment x day(ENV) effects
were tested by means of the PDIFF option and were declared
significant at P < 0.05. One YB cow was removed because of
adaptation complications: severe hypophagia and constant
standing.
Environmental conditions prior to arrival to ARC were not
documented. All cows of the same treatment were housed in the
same Saudi style barn cooled using Koral Kool systems and were
kept below 72 THI. It isn’t known whether conditions were over
68 THI. The recorded environmental conditions for the TN, HS,
and REC periods of the study are shown (Figure 2). As cows are
exposed to conditions above 68 THI, RR exceeds 60 [13] and RT
elevates above 38.5oC [3]. Due to these differences in parameters,
changes in physiology and performance measures are prevalent
between environmental groups Table 1.
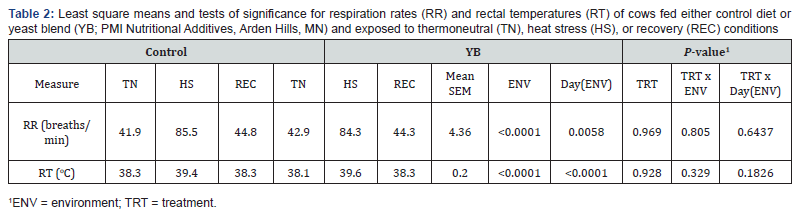
Mean RR and RT in CON and YB fed cows are shown in
Table
2. There was no difference in treatment for RR and RT. There is
conflicting data on the effect of treatment on RT. Baumgard et
al. [14] reported an increase in RT when cows were fed a feed
additive during HS. Other studies reported a reduction in RT
with supplementation during hyperthermia [9,15]. There was an effect of
environment (P<0.0001) for both these variables as cows
exposed to a THI above the threshold increases these parameters.
HS cows increased RR (42 breaths/min) and RT (1.32 oC) compared
to TN cows which indicates heat stress was achieved.
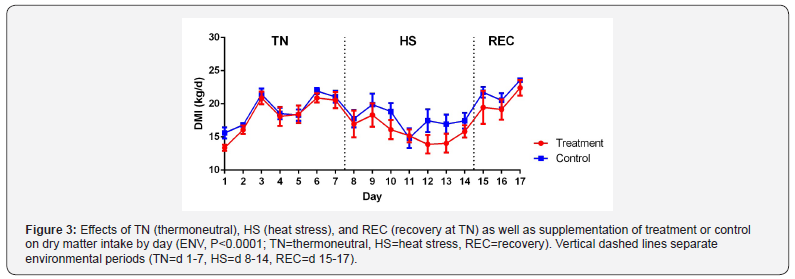
Feed intake, water intake, milk yield, and components are
reported in Table 3. Dry matter intake (DMI) increased in both
groups as cows adjusted to the facility during the TN period but
experienced a slight decrease due to feed heating during 2 days
of storage during TN period (Figure 3). DMI differed between TN
and HS (P=0.0007). Thermal stress has been well documented in
causing a decrease in DMI and how that drop in DMI may impact
milk yield. Previous heat stress research has shown an altered
endocrine profile that differs from nutrient restricted cows which
affect precursors of milk components, such as glucose [14,16,5].
There was no effect of TRT and TRT x ENV for DMI. Schingoethe et
al. [17] had similar findings in lactating Holsteins cows that were
fed supplementation of a yeast culture had no effect on DMI during
the summer. On the contrary, Wiedmeier et al. [18] suggested
that during periods of stress, fungal supplemental products
could be effective with increasing digestibility of structural
carbohydrates. This is supported by Moallem et al. [19] using a live
yeast supplementation which saw an increase in feed intake and
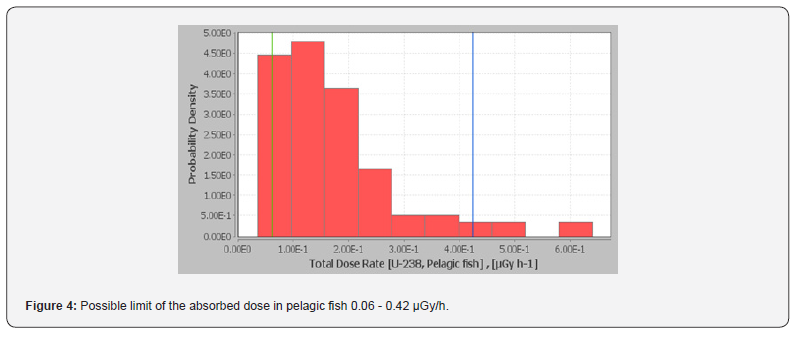
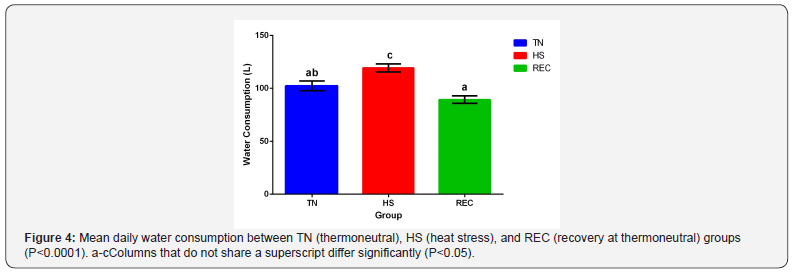
efficiency during the hot season. There was no difference in water
intake between treatment groups for each environmental phase
(Figure 4). Water intake increased during HS from TN (P=0.0005)
and decreased during REC from HS (P<0.0001). Murphy et al. [20]
reported similar water intake values in lactating Holstein cows.
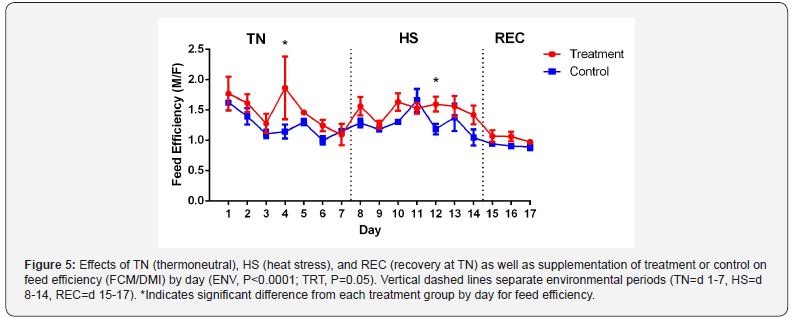
Cows fed YB had a higher feed efficiency (1.34±0.08 vs. 1.15±0.08,
P=0.05) compared to CON (Figure 5). There was also an effect of
ENV (P<0.0001) with feed efficiency increasing during HS and
decreasing during REC. Improvement in feed efficiency has been
reported in hyperthermic conditions with supplementation of feed
additives that contained live yeast and yeast culture [19,17]. YB
increased feed efficiency in general even though there was no ENV
effect. BW differed between TRT (P=0.0235) with YB cows having
lower BW than CON. There was an effect of ENV (P=0.0103) with
BW decreasing during periods of HS. The interaction between
ENV x TRT was not significant for BW.
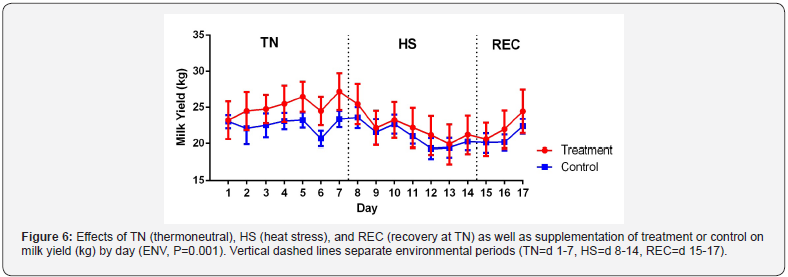
Milk and FCM yield differences weren’t detected between CON
and YB groups (Figure 6 and Table 3). These results are similar
to previous studies using feed additives containing yeast cultures
[17,21]. Other studies have shown an increase in milk yield with
yeast supplementation but it is correlated with greater DMI for
ruminants [22,23]. Milk yield declined during HS (P=0.002).
This decline in milk yield is only partially due to a decrease in
DMI which has been shown by Rhoads et al. [16]. Differences
between environments were found in FCM with TN being more
elevated than HS (P=0.02). There was no significant difference
in the following milk components: protein percentage, lactose
percentage, and SCC. Previous work by Hall et al. [10] with HS has shown an increase in SCC during the REC but there was no
significance observed in this study. The hypothesis is that during
heat stress, mammary epithelial cells sluff post HS and contribute
to the rise in SCC. Fat percentage had an effect of ENV (P=0.0129).
Milk fat percentage had no difference between TN and HS which is
surprising while there was fat depression observed during the REC.
Rhoads et al. [16] reported that climate-controlled HS cows don’t
have reduced milk fat percentages, unlike commercial dairies that
frequently experience fat depression during the summer months
due to supplementation with high concentrate diets [6]. Not much
work has been done during the REC and further research needs to
evaluate this in climate controlled cows.
Hormone and metabolite data are presented in Table 4. No
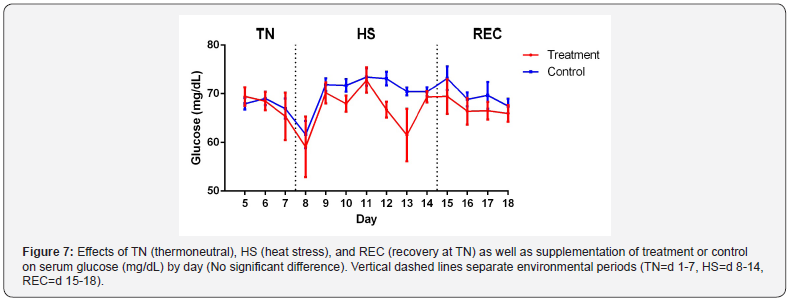
differences were detected in serum glucose due to TRT, ENV or
TRT x ENV (Figure 7). This is contrary to other findings [16,8].
Cowley et al. [24] had similar results and suggests that the glucose
homeostatic regulatory system is able to compensate for the
reduction of DMI. No significant differences in serum NEFA for
TRT, ENV or TRT x ENV were detected. Serum BHB did not have
any significant differences in TRT or TRT x ENV but an effect of ENV
was observed (P=0.0009). NEFA and BHB are a significant source
of energy for cows in a negative energy balance. BHB decreases
during HS according to Dale and Brody [25] but a decrease
during REC which has TN conditions is surprising. This suggests a
potential shift in postabsorptive changes in lipid metabolism since
ketones are a by-product of fatty acid oxidation and the cow now
favors glucose to lower heat production.
There was a difference in ENV for BUN (P<0.0001) and there
were no differences in effect for TRT or TRT x ENV. The increase of
BUN during HS is similar to previous studies [8,5]. BUN originates
from two sources: inefficient rumen ammonia incorporation
or deamination of amino acids as glucose precursors. Erasmus
et al. [7] found that yeast supplemented groups during HS had
improved rumen nitrogen balance which supports the increase
in urea-nitrogen in circulation. During periods of inadequate
nutrition, cows will undergo proteolysis in skeletal muscle to
support lactation [26]. BUN source could have derived from
muscle catabolism; however, BUN isn’t a good marker and either
3-methyl-histidine or creatine should be used which increase in
HS lactating cows [27,28].
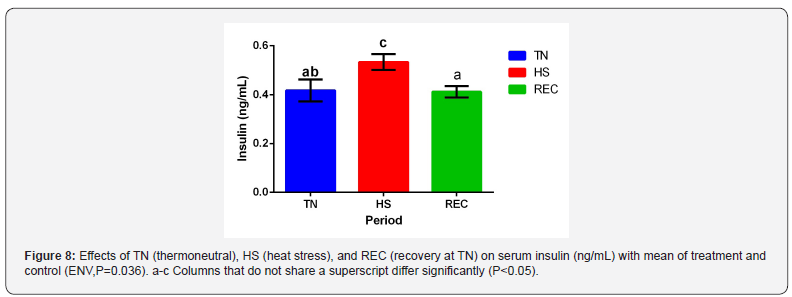
No difference was detected in serum insulin between TRT
groups and TRT x ENV. There was an effect of ENV (Figure 8)
with insulin increasing during the HS period (P=0.036). Elevated
insulin levels have been reported likewise during HS periods
[29,5]. This helps explain why there is no NEFA response due
to insulin’s antilipolytic properties [30]. Insulin also stimulates
protein synthesis and serves as an antiproteolyic hormone [31]
which supports the decrease in BUN.
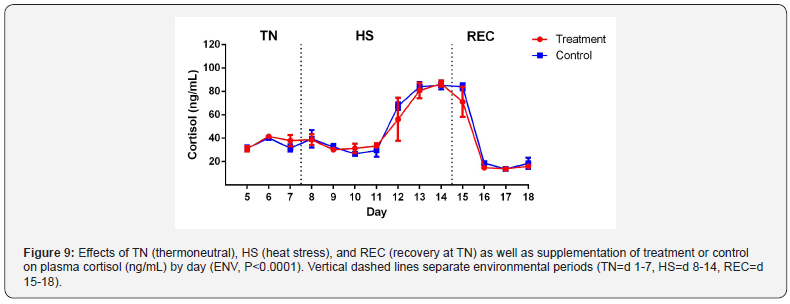
Plasma cortisol had no effect of TRT and TRT x ENV. As
expected, there was a significant impact of ENV on plasma cortisol
(Figure 9; P<0.0001). Plasma cortisol has been noted to have an
increase during acute but not chronic HS [32]. Interestingly, we
found no response during the acute phase for acclimation to HS
and an elevation during the chronic phase. Alvarez and Johnson
[33] reported cortisol spikes that ranged from minutes to hours
after an immediate insult of heat stress which would be on d
8. During acute heat stress, cows adjust cellular pathways to
mitigate the effects of heat stress and maintain homeostasis. This
is no longer needed once the acclimated phenotype and cows
have reached long term heat acclimation [34]. This supports the
decrease of cortisol during the acclimation period. One possibility
is the collection of blood samples in the AM may have missed
the spike in cortisol since cortisol levels peak in the afternoon.
Another possibility is that although similar THI was used to
previous studies conducted on HS, a difference in temperature and
humidity could have caused this since there can be the same THI
but different values for temperature and humidity
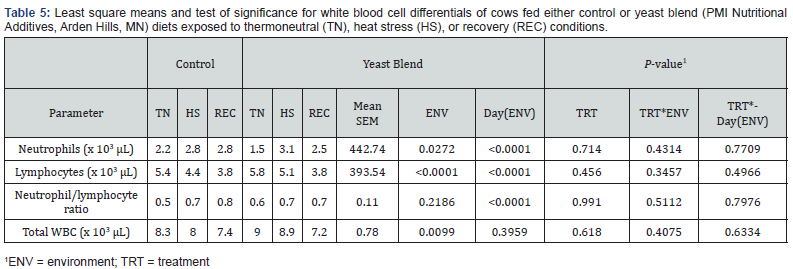
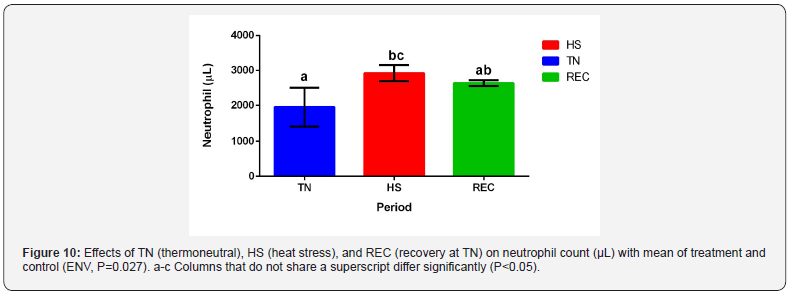
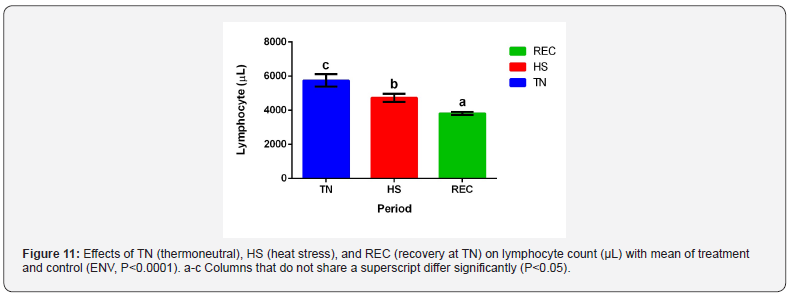
White blood cell differentials are shown in Table 5. Neutrophil
count increased during HS (Figure 10) with an observable
difference with an effect for ENV (P=0.02) but not for TRT and
TRT x ENV. Lymphocytes decreased during HS and REC (Figure
11; P<0.0001) while having no other differences in TRT and TRT
x ENV. Interestingly, there was no difference in the neutrophil to
lymphocyte ratio. Total white blood cell count was more elevated
during TN and decreased throughout the thermal periods
respectively (ENV, P=0.009). The results agree with stress induces
neutrophilia and lymphopenia [35,36-38,30].
In conclusion, supplementing mid-lactation Holstein cows
with YB beginning 14 days before and during exposure to
moderate HS showed an increase in some production responses
measured by feed efficiency. Feeding YB; however, did not result
in the mitigation of negative physiological responses associated
with HS. The mechanism behind the increase in feed efficiency
is unknown due to there being no glucose or DMI response to
treatment and further research should focus on it.
To Know more about Dairy & Veterinary Sciences
Click here: https://juniperpublishers.com/index.php