Agricultural Research & Technology
Summary
Sweet potato is an important food crop in the tropical, subtropical and temperate regions. It is essential for its economic value and the potential contribution to human health. The roots serve as a good source of vitamins, protein and carbohydrates. The response to in vitro regeneration of four genotypes P1P1 No.345, SP 9, N3 and Local white2 No. 274 were studied in this work. The establishment of an efficient sweet potato regeneration protocol using meristem bud through calli was obtained by using two types of cytokinins separately Benzyl-aminopurine and Kinetin at various concentrations (0.0,0.05,0.5, 1,0, and 1.5mg/l) plus a full-strength MS (Murashige and Skoog 1962). It was observed that the responses to BAP and kinetin were genotype dependent. The results showed that Kinetin 0.5mg/l resulted in the maximum callus production for accessions 345, SP9 and N3, while 0.05mg/l Kinetin produced the highest callus on 294. BAP 1.5mg/l significantly (P ≤ 0.05) performed best for shoots regeneration, in 345, SP9 N3 than the other treatments. The highest shoot regeneration for 294 occurred at 1.0mg/g BAP. Leaves regenerated best on Kinetin 0.05mg/l for the three accessions but 294 gave the highest percent regeneration on 1.5mg/l Kin. With respect to root production Kin1.5mg/l did extremely well in the absence of auxin.
Analysis of interactions between the sweet potato accessions and the cytokinins (BAP and Kin) for shoot regeneration indicated that generally acc. N3 had the best response for shoot regeneration and acc.294 the least. The study also focused on in vitro slow growth preservation using two growth retardants on two selected sweet potato varieties among the four that were used for regeneration N3 and SP9. Sucrose was varied at (0.0, 15, 20, 25 and 30g/l) and added to full strength MS. Treatments 0.0g/l and 30g/l sucrose were observed to be more suitable for slow growth in terms of shoot height, leaf numbers and number of nodes. Sorbitol was used as energy supplement in the full-strength MS at (0.0M, 0.2M, 0.4M, and 0.6M). Shoots of accession N3 and were cultured on MS containing varying treatments as sorbitol. 0.0M and 0.6m were the treatments that performed best for all the parameters considered regarding slow growth conservation.
Keywords:Sweet potato; Growth; Preservation; Sorbitol; Shoot height; Leaf numbers; Cytokinins; Auxin; Root production; Genotypes; Ipomoea batatas; Wheat; Rice; Maize; Potato; Barley; Cassava
Introduction
Sweet potato [Ipomoea batatas (L.) Lam] has its origin in South America [1] and the most important center of diversity is located in the area of Peru, Colombia and Ecuador [2]. It is a dicotyledonous root tuber crop which belongs to the family Convolvulaceae - commonly called morning glory [3]. Sweet potato is characterized by its starchy, luscious and tuberous storage roots. Though it is a perennial, it can be grown every twelve months by vegetative propagation using either storage roots or stem cutting [4]. According to Yoshiaki et al. [5], the crop can grow under fix carbon and stressful environmental conditions better than many other plant species. The cultivated species I. batatas includes plants that are very variable in morphology. And thousands of cultivars have been selected and cultivated in Latin America since ancient times and at present, the crop is cultivated throughout the tropics [6]. Sweet potato is a major staple food and income source in several regions of Africa [7]. The crop is becoming an important part of the diets of many households in Africa [8]. Owing to its versatility and adaptability, sweet potato ranks as the world’s seventh most essential food crop after wheat, rice, maize, potato, barley, and cassava, as it constitutes a considerable source of carbohydrate and carotene [9,10]. According to the Food and Agriculture Organization statistics [11], world production was 127 million tons of sweet potatoes majority of which came from China (105 million tons).
Sweet potato production is important for its nutritional and economic values in West Africa. Beta –carotene and some other very important elements are found in sweet potatoes which contribute to human health and nutrition for the present generation and generations yet to come [12]. The crop is mainly used in its primary state boiled, fried, or baked [13]. The young leaves and vine tips of sweet potato are widely consumed as a vegetable in some West African countries such as Liberia, Guinea and Sierra Leon as well as in North Eastern Uganda, and East Africa [14]. According to FAO [15], sweet potato leaves and shoots are a good source of vitamins A, C, and B2 and that regular intake can supply the recommended level of vitamin A to children less than 5 years of age [16]. Despite the significance of sweet potato production, it has been consistently faced with constraints such as diseases caused by pathogen infection of planting materials by pathogens [17], low yield, shortage of material from improved varieties, inappropriate preservation methods of planting material and difficulty in regeneration [18]. Efforts at developing disease free planting materials in recent times have focused on regeneration of plants from somatic embryo and in vitro selection techniques [19].
Tissue culture techniques have opened a new leading edge in agricultural science by addressing food security and agricultural production issues [20] and have led to more emphasis being placed on the use of biotechnological methods for genetic improvement of exacting traits in sweet potato.
Traditional planting methods using shoot tips for sweet potato are time consuming and labor intensive. If the parent plant is infected with diseases, they may be transmissible to the next generation, in spite of the application of fungicides or insecticides [21]. One of the main problems which result in the unattractiveness and low-income generation of this crop is the complexity in storage of planting materials. Viability of planting materials and freedom from plant pathogens can be addressed through tissue culture techniques. Tissue culture conditions, however, do not only vary for different crops but also for genotypes as well. There is, therefore, the need to determine the response of different sweet potato accessions to in vitro regeneration and identify appropriate conditions for slow growth in vitro preservation.
The objectives of this study are to:
i. Compare the relative responses of the four sweet potato accessions in different regeneration media.
ii. To study the response of two sweet potato accessions to different Osmotica as a first step in the development of in vitro preservation protocol for the four sweet potato accessions.
Materials and Methods
Experimental site
The study was carried out at the Tissue Culture Laboratory of Biotechnology and Nuclear Agriculture Research Institute (BNARI) of the Ghana Atomic Energy Commission (GAEC), Accra Ghana.
Experimental materials
Experimental materials were obtained from the green house at Biotechnology and Nuclear Agriculture Research Institute (BNARI). Four sweet potato accessions namely, P1P1 No.345, from East Africa, SP 9 from South Africa, N3 from Ghana, Local white2 No. 274 from Nigeria, and Boto3-036 No. 294 from Bonus/ germplasm bank. The lines of four sweet potato genotypes veins were grown in Polythene bags filled with sterile soil in the screen house forty-two days. The plants were watered twice a week with Nitrogen, phosphorus and potassium (NPK) solution. Shoot tips were excised, for meristems extraction and used explants source in the study.
Media preparation
The Murashige and Shoog (MS) basal medium supplemented with 30g/l sucrose, 0.1g/l myo-inositol, 0.05mg/l Bap , 0-5mg/l KIN or 0-4mg/l 2,4-D.Vitamines such as Thiamine 1mg/l Pyridoxine ,1mg/l Niotinic acid 1mg/l, Claforan 1mg/l, and CuS04 1mg/l Glycine at 2mg/l were used. The media were solidified with Phytagel 3.5g/l and PH was adjusted to 5.8 prior to autoclaving at 121˚C for 15mins. Laboratory Test tubes (20ml) were used for the experiment. Each test tube contained 15ml of the prepared media.
Explants sterilization protocol
Shoot tips of the green house grown accessions measuring 0.8-1.0cm in length were harvested with surgical blade and taken to the preparation room of the Laboratory in sterilized jars containing distilled water. This was done for each accession. The explants were washed several times and placed under running tap for 30mins. It was later carried to the laminar flow cabinet for sterilization. The explants were then immersed in 70% ethanol for 20 minutes and were rinsed several times in sterile distilled water
Data collection
Data were collected on the following: Number of explants that developed callus, number of explants that developed shoots, number of leaves per plant, and number of roots per plant.
Data analysis
All results were subjected to analysis of variance (ANOVA). GENSTAT (version, 12.0) statistical package was use for data analysis. The means were separated using least significant difference (LSD) at 5% level of significance.
Results and Discussion
Objective I
Compare the relative responses of the four sweet potato accessions in different regeneration media.
Effect of BAP or kinetin on callus production from meristems of sweet potato accession 294:
BAP applied at a concentration of 0.05mg/l showed the highest callus production of 56.7% in the fourth week (Table 1). This was significantly different from the observed percent callus production in the sixth and eighth weeks, and also significant at (P ≤ 0.05) from the lowest of (45.0%) obtained in the second week. The highest mean callus production was 71.5% which occurred on 0.5mg/l BAP at the fourth week and was maintained through the eight weeks period. On BAP at 1.0mg/l gave the highest callus production percent of 63.4 occurring the first week and declined to 56.7 in the six weeks. 1.5mg/l BAP, gave its highest mean callus production of 50.7% within the second and fourth week, but declined to 45.0% in the six seventh weeks. All differences between the different hormone concentrations were significant at (P ≤ 0.05).
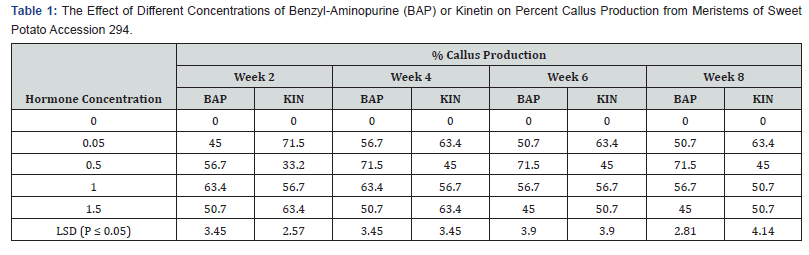
Percent callus production was generally higher in kinetin treatments at the lowest (0.05) and the highest (1.5mg/l) concentrations than the other Kinetin concentrations. (PLATE1 A, B, C and D) show the callus production for both Kinetin and BAP. The highest callus produced with Kinetin supplementation was 71.5% observed on 0.05mg/l Kin. This occurred in the second week. However, on 1.0mg/l Kin the highest callus production was 56.7% from week two to week six and subsequently declined to 50.7% at week eight. On Kinetin supplemented media the lowest callus production which Also, at 1.5mg/l Kin, 63.4% was the highest callus production from the second week to the fourth week but reduced to 50.7% from week six to week eight. From 33.2% to 45% occurred on treatment supplemented with 0.5Kin. BAP performed best (71.5%) at 0.5mg/l, while kinetin performed best at (71.5%) at 0.05mg/l.
Effect of varying concentrations of BAP or kinetin on shoot production in accession 294:
Table 2 shows percent shoot production in accession 294 for the sixteen weeks culture period. Shoot initiation occurred in the tenth week on all hormone treatments except the control. The production of shoots varied with cytokinin type (BAP and Kin) as well as concentration and culture period. With 0.05mg/l BAP the highest percent shoot produced (39.2%) was obtained in the sixteenth week. Whiles in the case of 0.5mg/l BAP supplemented treatment, shoot production started at the tenth week with as high as 33.2% initiation which was maintained through to fourteenth week, and the highest shoot production of 45.0% occurred in the sixteenth week. BAP applied at 1.0mg/l also showed the highest shoot production of 50.7% in the sixteenth week and the lowest of 18.4% in the tenth week.
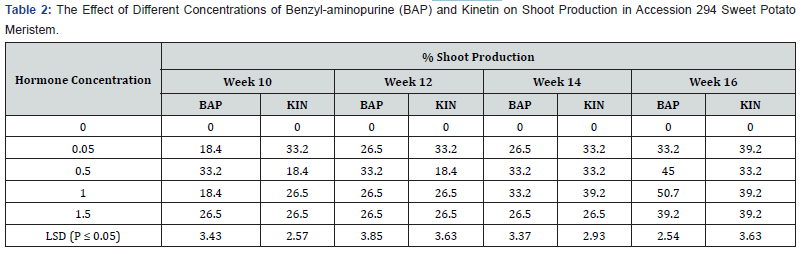
BAP supplemented treatment of 1.5mg/l gave the highest shoot production percent of 39.2% in the sixteenth week starting with of 26.5% from week ten to week fourteen. For both types of cytokinins the highest mean shoot regeneration differed significantly from the other treatments at (P ≤ 0.05). The control 0.0mg/l, completely failed to induce shoot regeneration during the sixteen weeks of culture. In the cases of kinetin treatments, 0.05mg/l Kinetin produced it highest shoot regeneration percent of 39.2% in the sixteenth week starting at 33.2% from week ten to week fourteen. Treatment containing Kin 0.5mg/l also showed the highest mean of 33.2% during the fourteen weeks and the lowest of 18.4% beginning from the tenth week to the twelve weeks. With 1.0mg/l and 1.5mg/l kinetin supplemented treatments a similar highest mean shoot production of 39.2 % was observed in week sixteen. With the exception of 0.5mg/l Kin which produced the lowest shoot regeneration of 33.2%, all the other kinetin supplemented treatments produced a maximum of 39.2% shoot production during the sixteen weeks culture period. Generally, percent shoot production at the end of the sixteen weeks of culture was batter on BAP than Kinetin supplemented media. The performance of the explants on 0.5mg/l Kin was significantly lower than all the other treatments except the control.
Effect of varying concentrations of BAP or kinetin on shoot production in accession 294:
Table 3 shows percent shoot production in accession 294 for the sixteen weeks culture period. Shoot initiation occurred in the tenth week on all hormone treatments except the control. The production of shoots varied with cytokinin type (BAP and Kin) as well as concentration and culture period. With 0.05mg/l BAP the highest percent shoot produced (39.2%) was obtained in the sixteenth week. Whiles in the case of 0.5mg/l BAP supplemented treatment, shoot production started at the tenth week with as high as 33.2% initiation which was maintained through to fourteenth week, and the highest shoot production of 45.0% occurred in the sixteenth week. BAP applied at 1.0mg/l also showed the highest shoot production of 50.7% in the sixteenth week and the lowest of 18.4% in the tenth week.
BAP supplemented treatment of 1.5mg/l gave the highest shoot production percent of 39.2% in the sixteenth week starting with of 26.5% from week ten to week fourteen. For both types of cytokinins the highest mean shoot regeneration differed significantly from the other treatments at (P ≤ 0.05). The control 0.0mg/l, completely failed to induce shoot regeneration during the sixteen weeks of culture. In the cases of kinetin treatments, 0.05mg/l Kinetin produced it highest shoot regeneration percent of 39.2% in the sixteenth week starting at 33.2% from week ten to week fourteen. Treatment containing Kin 0.5mg/l also showed the highest mean of 33.2% during the fourteen weeks and the lowest of 18.4% beginning from the tenth week to the twelve weeks. With 1.0mg/l and 1.5mg/l kinetin supplemented treatments a similar highest mean shoot production of 39.2 % was observed in week sixteen. With the exception of 0.5mg/l Kin which produced the lowest shoot regeneration of 33.2%, all the other kinetin supplemented treatments produced a maximum of 39.2% shoot production during the sixteen weeks culture period. Generally, percent shoot production at the end of the sixteen weeks of culture was batter on BAP than Kinetin supplemented media. The performance of the explants on 0.5mg/l Kin was significantly lower than all the other treatments except the control.
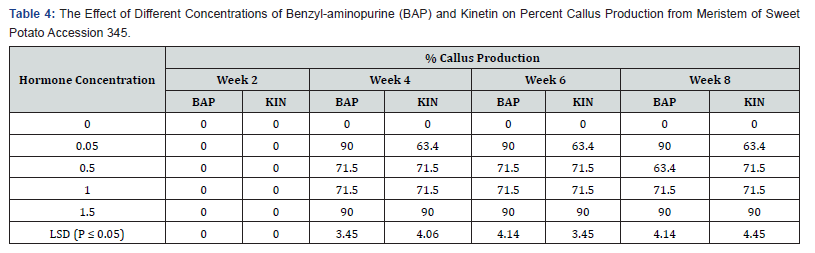
Effect of various concentrations of BAP or kinetin on callus production from meristem of sweet potato accession 345:
Table 4 shows the effect of varying concentrations of BAP and Kinetin on callus production in accession 345. Media without hormone supplement did not produce any callus in Acc. 345 meristems. Callus production begun in the fourth week for both Kin and BAP supplemented media at 0.05mg/l to 1.5mg/l with the highest percent callus production of 90.0% beginning from the fourth week to the eighth week, 0.5mg/l and 1.0mg/l of both BAP and Kin also had the same callus production of 71.5% with the acception of 0.5mg/l BAP treatment which produced 63.4% callus week eight. Kinetin at 0.05mg/l gave the highest percent callus production of 63.4%, starting from the fourth week. Whiles 1.5mg/l KIN showed 90.0% percent callus production from week four through week six. The difference in callus production on 0.05mg/l BAP and 1.5mg/l BAP were not significant as the difference between treatments with 0.5mg/l and 1.0mg/l BAP accept at week eight. In the case of Kinetin supplemented treatments callus production were significantly different (P ≤ 0.05) between 0.05mg/l and 1.5mg/l Kin, but there was no significant difference between the 0.5mg/l and 1.0mg/l Kinetin supplemented treatments.
Effect of varying concentrations of BAP or kinetin and culture period on shoot production from meristem of sweet potato accession:
As shown in table 5, the control treatment (0.0mg/l cytokinin) did not produce any shoots. On meristem of acc 345, MS medium supplemented with BAP at 0.05mg/l induced the highest percent shoot production of 71.5% during the tenth week but shoot production gradually declined to 45.0% in the sixteen weeks. Media supplemented with 0.5mg/l BA induced the highest percent shoot production of 63.4% in the tenth week and steadily dropped to 33.2 in the sixteen weeks. Also, BAP 1.0mg/l had the highest percent shoot of 50.7%, occurring in the tenth week as well. Whiles 1.5mg/l Kin showed the highest shoot production of 63.4 % which was observed in the sixteen weeks. A similar trend was observed in Kinetin supplemented treatments with the highest number of shoot regeneration occurring in the tenth week but declining steadily to the lowest in the sixteenth week with the exception of treatments involving 1.5mg/l Kinetin supplementation where shoot production percentage was lowest (56.7%) at week ten after culture and stayed almost constant until the sixteenth week and increased to 63.4%.
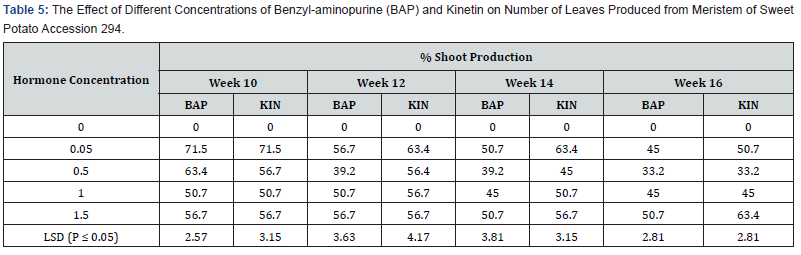
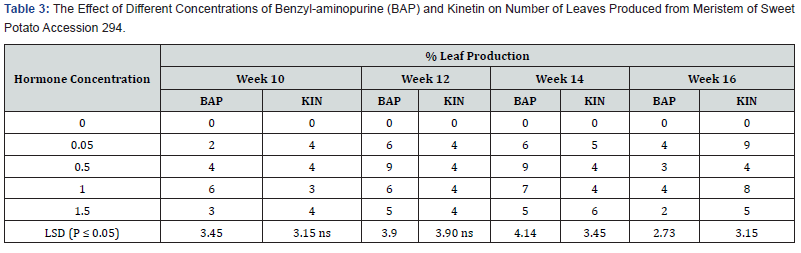
Effect of BAP or kinetin on number of leaves in accession 345:
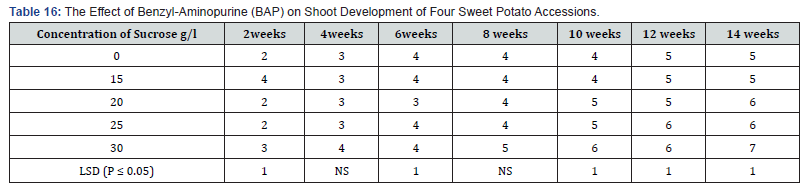
No leaves were formed on the hormone free, control treatment (Table 6). However, on the media supplemented with different concentrations of BAP, showed different levels of leaf production on the shoots produced. The highest number of leaves per plant was 4.0 on media supplemented with BAP 0.05mg/l, 1.0 mg/l and 1.5mg/l occurred in the tenth week. The lowest number of leaves produced in these treatments was 2.0. In the treatment supplemented with BAP 0.5mg/l the highest leaves per shoot produced was 5.0 in the tenth week but it dropped to 3.0 in the sixteenth week. Leaf production in BAP supplemented treatments was generally higher in the 0.5mg/l, but the difference was not significant. KIN, at 0.05mg/l had the highest number of leaves per shoot of 9.0 at the end of the sixteenth week followed by kinetin at 1.0mg/l with 8.0 leaves per shoot at the sixteenth week and treatment 1.5mg/l with 5.0 leaves per shoot at sixteenth week in that order. Contrary to leaf production in the BAP supplemented treatments the numbers of leaves per shoot in Kinetin supplemented treatments was generally higher at week sixteenth and the difference in leaf production under the various Kinetin concentrations were significant (P ≤ 0.05) at the tenth, fourteenth and sixteenth week.
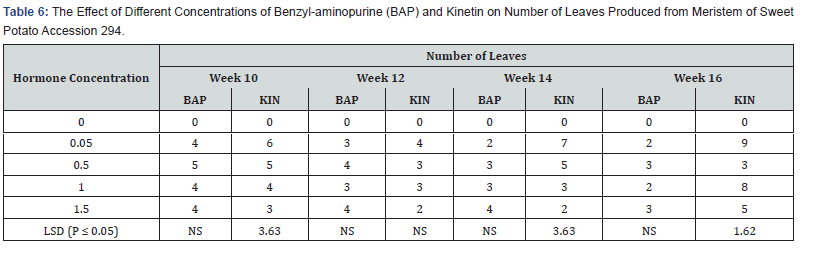
Influence of varying concentrations of BAP or kinetin on regeneration of sweet potato accession SP9:
The results of callus production on SP9 from week two to week eight are shown in table 7. Callus production did not begin until the sixth week, while in the cases of hormone free media no callus was produced. BAP 0.05 mg/l had the highest callus production of 71.5% at week six and was maintained through to the eighth week. 0.5 mg/l and 1.5mg/l BAP had 90.0% callus production in the six weeks up to the eighth week. 1.0mg/l BAP had the highest callus production of 63.4% which also started form the six week and continued to the eighth week. Kinetin showed similar trend to BAP. 0.05mg/l and 1,5mg/l KIN produced maximum callus percentage of 71. 5% at week six. Treatment 0.5 mg/l KIN produced the highest percentage of 90.0%, which occurred in the six week and was also maintained till the eighth week. Treatment 1.0mg/l KIN showed the lowest callus percentage of 63.4 % from week six till the eighth week. There were significant differences in both hormones at the various treatments’ levels.
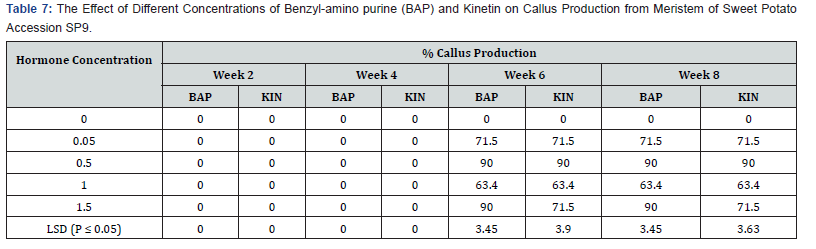
Effect of different levels of BAP or kinetin on shoot regeneration in accession SP9:
As shown in table 8 shoot productions varied among the concentrations of the two plant growth regulators that were used. Media without hormone supplement did not produce any shoot. Shoot production on both BAP and Kin supplemented media started in the tenth week for all treatments. Among the BAP treatments 0.05mg/l induced the lowest shoot production of 45.0 % in the sixteenth week. The treatment 1.5 mg/l BAP supplemented had the highest shoot production of 71.5% in the tweleth and fourteenth week followed by a decline in shoot production of 63.4 % at week sixteen. This was followed by the treatment containing 1.0mg/l BAP with shoot production of 56.7% at week sixteen. Kinetin supplemented media also showed differences among treatments. At week sixteenth the highest percent shoot production (63.4%) occurred in the 0.5mg/l Kin supplemented treatment. This was followed by the treatment 1,5mg/l Kin with 56. 7% shoot production, the treatment containing 1.0mg/l Kin produced the lowest percent shoot production (45.0%). The differences between shoot productions on the various concentrations on the two plant growth regulators were significant (P ≤ 0.05).
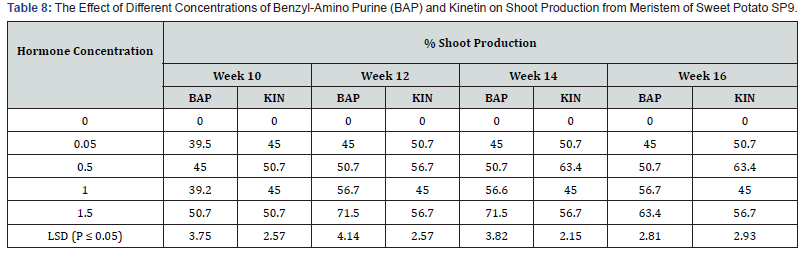
Effect of different levels of BAP or Kinetin on shoot regeneration in accession SP9: As shown in table 9 shoot productions varied among the concentrations of the two plant growth regulators that were used. Media without hormone supplement did not produce any shoot. Shoot production on both BAP and Kin supplemented media started in the tenth week for all treatments. Among the BAP treatments 0.05mg/l induced the lowest shoot production of 45.0 % in the sixteenth week. The treatment 1.5 mg/l BAP supplemented had the highest shoot production of 71.5% in the twelveth and fourteenth week followed by a decline in shoot production of 63.4 % at week sixteen. This was followed by the treatment containing 1.0mg/l BAP with shoot production of 56.7% at week sixteen. Kinetin supplemented media also showed differences among treatments. At week sixteenth the highest percent shoot production (63.4%) occurred in the 0.5mg/l Kin supplemented treatment. This was followed by the treatment 1,5mg/l Kin with 56. 7% shoot production, the treatment containing 1.0mg/l Kin produced the lowest percent shoot production (45.0%). The differences between shoot productions on the various concentrations on the two plant growth regulators were significant (P ≤ 0.05).
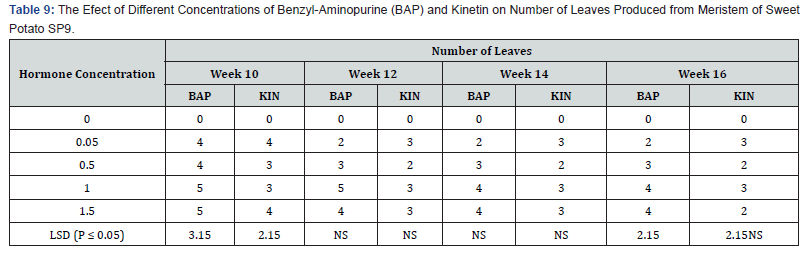
Regeneration in sweet potato accession N3 as influenced by BAP and kinetin concentrations:
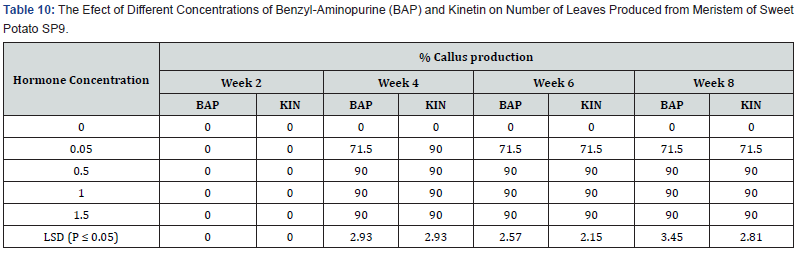
Table 10 shows no callus production in the control 0.0mg/l. For all the other hormone levels, callus production commenced in the fourth week after culture. 0.05mg/l BAP treatment showed a highest callus production of 71.5%. The remaining three concentration levels of both BAP and Kinetin very high and equal (90%) levels of callus production at week four and was maintained throughout the eight weeks of callus production. The differences between callus production 0n 0.05mg/l BAP and 0.05mg/l Kin and the other three application levels of both hormones were significant at (P ≤ 0.05).
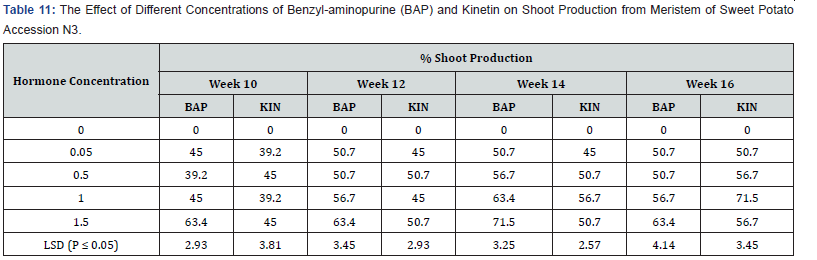
Influence of varying concentrations of BAP or kinetin on shoot production in accession N3:
No shoot production occurred on the hormone –free (control) media (Table 11). With the BAP supplemented treatments, treatment 1.5mg/l showed the highest shoot production (63,4%) at week ten while BAP applied at 0.5mg/l produced the lowest shoot percentage (39.2%). At the end of week sixteenth, BAP 1.5mg/l treatment still showed the highest shoot production rate of (63.4%) among the BAP treatments. Shoot production reached the peak of 71.5 at week fourteen. Shoot numbers in all BAP treatments at the fourteenth week declined slightly at week sixteenth. In the case of Kinetin supplemented treatments, the highest percentage shoot production (45.0%) at week ten and week twelve (50.7%) was observed in treatments containing 0.5mg/l and 1.5mg/l Kinetin. At the fourteenth and sixteenth weeks shoot production in the treatment containing 1.0mg/ kinetin increased to the percentage of 71.0 %. Shoot production increased steadily (In treatment 1.0mg/l Kin) from 39, 2 in week ten to 71.5% at the end of the sixteenth week. The differences between all the treatments were significant at (P ≤ 0.05).
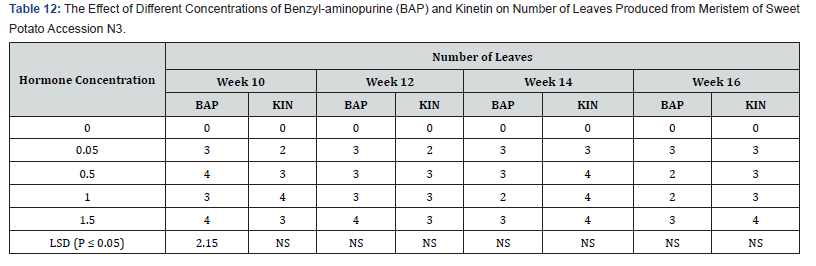
Leaf numbers per shoot in accession N3 as influenced by different concentrations of BAP or kinetin:
Hormone free medium failed to regenerate and subsequently produced no leaves (Table 12). All the other hormone supplemented treatments showed some leaf production at week ten. BAP 0.05mg/l and 1.5 mg/l had number of leaves of 3.0 and 4.0 respectively at week ten. At the end of week sixteen the highest number of leaves on BAP supplemented treatments, was observed on treatments 0.05mg/l and 1.5mg/l BAP. The highest number of leaves per shoot on the Kinetin supplemented treatments was also 4.0. This occurred on treatments supplemented with 1.0mg/kinetin at week ten and reduced to 3.0leaves per shoot at week sixteen, while treatments containing 1.5mg/l Kin produced 3.0 leave per shoot at week ten but increased to 4.0 by the sixteenth week. With the exception of leaf production at week ten in varying Kinetin concentrations which were significantly different from the control (P ≤ 0.05). The differences between all the other treatments over the experimental period were not significant.
Influence of genotypes (accessions) and BAP concentrations on shoot generation:
The mean number of shoot regenerated in the four accessions at various BAP concentrations ranged between 33.6 and 44. 3 (Table 13). The highest mean shoot regeneration occurred in Accession N3 followed by accession SP9 but the difference between the two were not significant. Significant (P ≤ 0.05) differences, however, occurred between shoot production in the two named accessions on one hand and in accessions 294 and 345 on the other. The various BAP concentrations used also produced significant (P ≤ 0.05) differences in shoot regeneration. BAP concentrations 1,0mg/l and 1.5mg/l produced significantly higher shoot regeneration than the other of BAP concentrations. Significant interactions were also observed between accessions and BAP concentrations.
Influence of genotypes (accessions) and Kin concentrations on shoot generation:
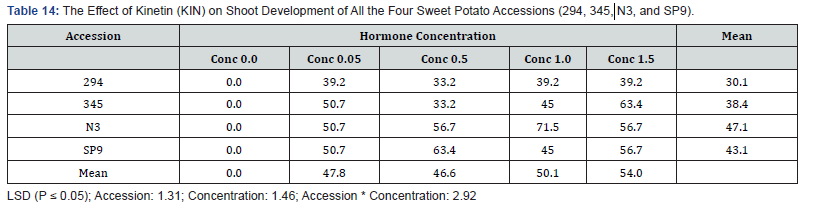
The interactions between the various Kinetin levels and accessions of sweet potato are shown in (Table 14). MS media without plant regulator supplement did not produce any shoot. Accession 294 cultured on the following concentrations (0.05mg/l, 0.5mg/l, 1.0mg/l and 1.5mg/l Kin) produced a mean percent shoot regeneration of 30.1%. Accession 345 had a mean percent shoot of 38.4% and was significantly different from accession 294 301%. Similarly accession N3 with the highest percent shoot regeneration of 47.1% and accession SP9 within second highest mean shoot number of (43.1) showed significant (P ≤ 0.05) difference in percent shoot regeneration. Significant differences were observed between the four accessions, hormone concentrations as well as the interaction between them.
Objective II
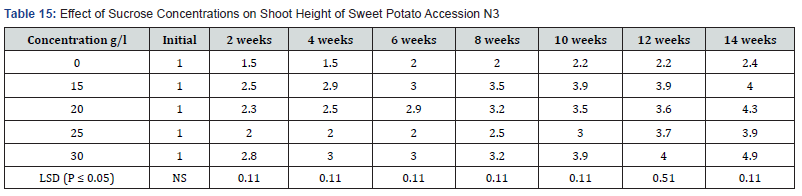
To study the response of two sweet potato accessions to different osmotica as a first step in the development of in vitro preservation protocol for the four sweet potato accessions. Table 1 shows shoot growth in centimeter (cm) on MS supplemented with varying concentrations of sucrose. MS medium containing 0.0mg/l sucrose produced the shortest plant height of (2.4cm) at the end of the fourteenth weeks of culture. The tallest plants were produced by the highest sucrose concentration 30g/l (which is the normal sucrose concentration for in vitro sweet potato culture). 15g/l, 20g/l and 25g/l sucrose levels did not produce significant differences in shoot height. However, the differences in shoot height between at 0.0g/l sucrose and the rest of the treatments were significantly different at (P ≤ 0.05).
The influence of varying sucrose concentrations on number of leaves and nodes developed from shoot of sweetpotato accession N3:
Table 15 shows the number of nodes that developed per plant. There was no increase in node numbers during the first four weeks of culture in all the sucrose treatments. Node started developing in the six weeks after culture. 0.0mg/l sucrose had four nodes in the fourteenth week. Treatment 1.5mg/l sucrose and 30mg/l sucrose produced two nodes in the sixth week which increased to seven nodes at week fourteen. Treatment 20mg/l sucrose also began node development with three nodes. While treatment containing 25mg/l sucrose produced three nodes in the six week and by the fourteenth week increased to seven. There were no significant differences between treatments with regard to rate of increase in nodes, as well as total number of nodes developed at the end of the experiment
The Effect of sucrose concentrations on leaves numbers per plant of sweet potato accession N3:
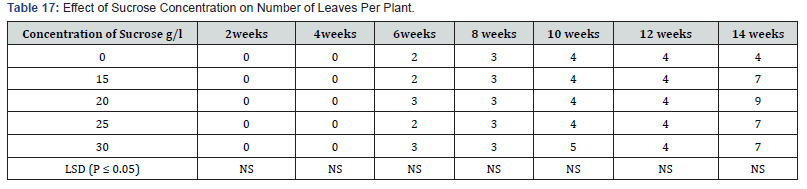
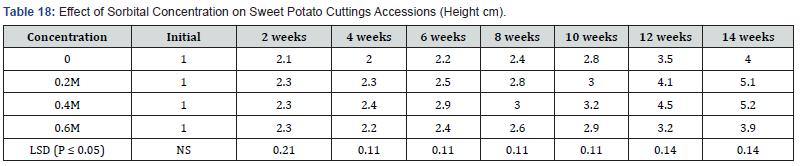
Significant differences were observed in number of leaves produced per plant (P ≤ 0.05). The number of leaves per plant increased as the culture period increased. Leaf numbers in culture containing various sucrose concentrations also showed increase with concentrations (Table 16). Treatment containing 0.0mg/l and 15mg/l sucrose had the same number (5) leaves per plant in the fourteenth week. Also, treatments 20mg/l and 25mg/l also had the same number of leaves six, per plant in week fourteen. Sucrose at 30mg/l supplement produced seven leaves per plant during the fourteenth week. Leaf production in the various treatments showed significant differences at (P ≤ 0.05) except for weeks four and eight. Influence of varying concentrations of sorbitol on shoot height in sweet potato accession SP9. Media supplemented with sorbitol, gradually increased shoot height of the in vitro cultured plants over a period of fourteen week in Table 17. Treatment 0.6M sorbitol produced plant with the shortest height of 3.9cm in week fourteen, and were followed by 0.0 M (hormone free) media with shoot height of 4.0 cm. Plants on treatment .0.2 M sorbital also increased in height to 5.1 cm while the treatment containing 0.4M sorbital produced shoot height of 5.2cm at the fourteen weeks. There were significant differences (P ≤ 0.05) between treatments from the second to the fourteenth week.
The influence of varying sorbitol concentrations on number of nodes developed from shoot of sweet potato accession SP9:
Table 18 shows the number of nodes that were developed on varying concentration of sorbitol. During the second and fourth week, nodes were formed on all treatments. The concentration 0.0M sorbitol produced one node in week six. While it subsequently increased to five by the fourteenth week. Treatment 0.2M sorbitol produced two nodes in the six weeks. The node number also increased to nine by week fourteen. Treatment 0.4M sorbitol developed eight nodes in the fourteenth week while 0.6M sorbitol also developed six nodes per plant at the end of the experiment. There were significant differences (P ≤ 0.05) in number of nodes per plant in the various treatments starting from the six weeks to week fourteen. Again, the lowest node number (5) was realized on the 0.0M (control), followed by 0.6M treatment with six nodes per plant.
The influence of varying sorbitol concentrations on number of nodes developed from shoot of sweet potato accession SP9:
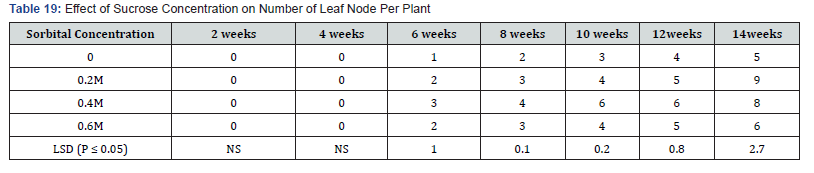
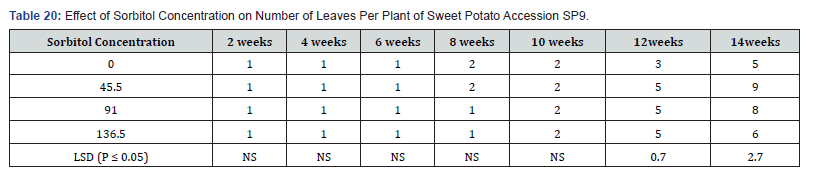
In Table 19,20 each sorbitol level produced one leaf per node from the second week to the sixth week. Leaf number increased in the treatment containing 0.2 M sorbitol at eight weeks but remained at one leaf per shoot for treatment 0.4M and 0.6 M sorbitol. At the tenth week all the treatments produced two leaves per shoot. At the end of the fourteenth week, the control had leaf number of five per shoot followed by treatment 0.6M with six leaves per shoot but, differences between them were not significant. Treatments 0.2M and 0.4M sorbitol produced the highest number of leaves per shoot of nine and eight respectively.
Conclusion
The present study has shown that sweet potato genotypes can be regenerated and conserved in vitro. However, there were differences between accessions in their response to the regeneration medium used in the study. The best plant growth regulators in combination with MS identified for callus production was full strength MS supplemented with 0.5mg/l Kinetin, this was the most ideal for all the accessions. The most suitable hormone type and concentration for shoot regeneration identified in this study was full MS with 1.5mg/l benzyl-aminopurine (BAP), while leaf regeneration was obtained to be most efficient on BAP 1.0mg/l plus full MS and root production was better on 1.5mg/l Kinetin.
In the case of in vitro slow growth conservation, the following treatments are promising. 0.0g/l sucrose that is media without sucrose supplementation. Sorbitol 0.6 M plus MS without sucrose performed well as MS without both sorbitol and sucrose supplementation. Plantlets obtained through the regeneration processes and plantlets subjected to the treatment’s conservation performed very well under natural environmental condition. Therefore, the regeneration protocol could be used for in vitro multiplication of sweet potato.




No comments:
Post a Comment